Enol facts for kids
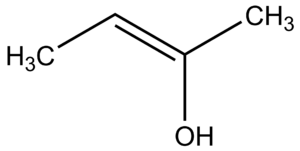
An enol is a special type of alkene molecule. Imagine a molecule with a double bond between two carbon atoms. An enol has an OH group (that's an oxygen atom connected to a hydrogen atom) attached to one of these carbon atoms that are part of the double bond.
If the hydrogen atom on the oxygen is taken away, the enol changes into something called an enolate.
Enols are like shape-shifters for other molecules called ketones or aldehydes. These different shapes are called tautomers. Tautomers are molecules that are almost identical, but a hydrogen atom has moved to a different spot. It's like moving a single LEGO brick on a structure to make a slightly different shape.
Scientists can easily make enols from ketones or aldehydes by using a special chemical helper called a base. Enols are not very stable, meaning they don't stay in their enol shape for long. This is because the C=O bond (carbon double-bonded to oxygen) in ketones and aldehydes is stronger than the C=C bond (carbon double-bonded to carbon) in enols.
Even though they are unstable, enols can take part in many interesting chemical reactions. A famous example is the aldol reaction, which is important in making new, larger molecules.
Contents
Making Enols: Two Ways
When you make an enol from a ketone, there are often two different places where the enol can form. Think of it like having two different doors to exit a room. Scientists need to choose which "door" or side they want the reaction to happen on.
The Easier Side (Kinetic Enolate)
The side of the ketone that has fewer other atoms or groups attached to it is usually the easiest to turn into an enol. This can be done at a low temperature and by using a "bulky" base. A bulky base is a large molecule that helps the reaction happen more easily at the less crowded spot. This type of enol is called the kinetic enolate. It forms quickly because it's the easiest path.
The Harder Side (Thermodynamic Enolate)
The other side of the ketone, which has more atoms or groups attached, is harder to turn into an enol. To make an enol on this side, you usually need higher temperatures. This type of enol is called the thermodynamic enolate. It's more stable in the long run, even though it takes more energy to create.
Images for kids
-
Conversion of ascorbic acid (vitamin C) to an enolate. This shows how electrons move when a stable molecule changes into an enolate.
See also
 In Spanish: Enol para niños
In Spanish: Enol para niños
 | Chris Smalls |
 | Fred Hampton |
 | Ralph Abernathy |