Aldehyde facts for kids
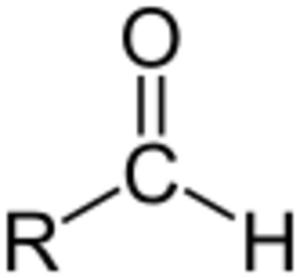
An aldehyde is a type of organic compound. Think of organic compounds as molecules that are mostly made of carbon and hydrogen. Aldehydes have a special part called a formyl group. This group is like a small building block within the molecule.
A formyl group has a carbon atom that is connected to an oxygen atom with a double bond. This same carbon atom is also connected to a hydrogen atom and another part of the molecule, which we call an "R group" or a "side chain." The side chain is just the rest of the molecule.
Aldehydes are different from similar compounds called ketones. In an aldehyde, the formyl group is always at the very end of the molecule. In ketones, this group is usually found in the middle. Aldehydes are very common in organic chemistry. Many things that have a strong smell, like certain fragrances, are actually aldehydes!
Contents
What Aldehydes Look Like
Aldehydes have a carbon atom that is double-bonded to an oxygen atom. This special connection makes them slightly polar. Being polar means one part of the molecule has a slightly positive charge and another part has a slightly negative charge.
This polarity gives aldehydes some important features. For example, smaller aldehydes can easily dissolve in water.
Naming Aldehydes
Official Names (IUPAC)
The official way to name aldehydes comes from the IUPAC. These names help scientists around the world understand exactly which molecule they are talking about.
Here's how they are usually named:
- If an aldehyde doesn't have carbon rings, its name comes from the longest chain of carbon atoms that includes the aldehyde group. You take the name of the parent alkane (a simple carbon chain) and change its ending from -e to -al.
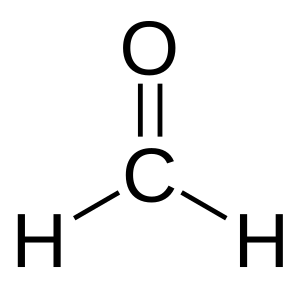
- For example, HCHO is named methanal because it's like methane.
- CH3CH2CH2CHO is named butanal because it's like butane.
- If a -CHO group is attached to a ring of carbon atoms, you add -carbaldehyde to the end of the ring's name.
- For example, C6H11CHO is called cyclohexanecarbaldehyde.
- If there are other important groups in the molecule, you might add formyl- to the beginning of the name.
Where the Names Come From
The word aldehyde comes from Latin. It's a shortened version of alcohol dehydrogenatus, which means "dehydrogenated alcohol." This name was created by a scientist named Justus von Liebig.
The term formyl group also comes from Latin or Italian. The word formica means "ant." This is because formic acid, which is related to the formyl group, was first found in ants!
Properties of Aldehydes
Aldehydes have many different properties, and these can change a lot depending on the rest of the molecule.
- Solubility: Smaller aldehydes, like formaldehyde and acetaldehyde, can completely dissolve in water.
- Smell: Many aldehydes have very strong smells.
- Stability: Aldehydes can break down if they are left in the air for too long.
Some important aldehydes, like formaldehyde and acetaldehyde, can link together to form long chains. This process is called polymerization.
Aldehydes in Nature
Many aldehydes are found in essential oils. These oils are what give plants their unique smells. For example, the smells of cinnamon, cilantro, and vanillin (vanilla) all come from aldehydes!
How Aldehydes React
Aldehydes are very reactive, meaning they easily take part in many chemical reactions.
- In Industry: Aldehydes are used to make many useful things. They help create plasticizers (which make plastics more flexible), polyols, and different types of alcohols.
- In Biology: In living things, aldehydes are used in important processes, like helping to form sugars.
Dialdehydes
A dialdehyde is a special type of molecule that has two aldehyde groups. The names of dialdehydes often end with -dial or -dialdehyde. Sometimes, they are named after a similar acid. For example, butanedial is also known as succinaldehyde, because it's similar to succinic acid.
Examples of Aldehydes
- Methanal (also known as formaldehyde)
- Ethanal (also known as acetaldehyde)
- Propanal (also known as propionaldehyde)
- Butanal (also known as butyraldehyde)
- Benzaldehyde
- Cinnamaldehyde
- Tolualdehyde
- Furfural
- Retinaldehyde
Examples of Dialdehydes
- Glyoxal
- Malondialdehyde
- Succindialdehyde
- Glutaraldehyde
- Phthalaldehyde
Uses of Aldehydes
Formaldehyde is the most produced aldehyde in the world. About 6 million tons are made each year! It's mainly used to create resins when mixed with other chemicals like urea, melamine, and phenol. A famous example is Bakelite, an early type of plastic.
Another commonly made aldehyde is butyraldehyde, with about 2.5 million tons produced annually. While a lot of acetaldehyde used to be made, less is produced today. This is because many chemicals that were once made from acetaldehyde are now produced in other ways.
Related pages
See also
 In Spanish: Aldehído para niños
In Spanish: Aldehído para niños
|