Polystyrene facts for kids
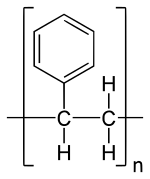
Polystyrene is a common type of plastic. It's a polymer, which means it's made of many small units linked together. This plastic is used for many things, especially packaging. It is made from a liquid called styrene, which comes from petroleum. Polystyrene is one of the most used plastics in the world.
Unlike some materials, polystyrene doesn't break down easily in nature. It can last for hundreds of years. Many environmental organisations want to limit how much foamed polystyrene is used, especially for takeout food. People are also looking for other materials to use instead of polystyrene foam in restaurants.
Quick facts for kids Polystyrene |
|
|---|---|
 |
|
 |
|
| IUPAC name | Poly(1-phenylethene) |
| Other names | Thermocol |
| Identifiers | |
| Abbreviations | PS |
| CAS number | |
| Properties | |
| Molecular formula | |
| Molar mass | 0 g mol-1 |
| Density | 0.96–1.04 g/cm3 |
| Melting point | |
| insoluble | |
| Solubility | Soluble in acetone |
| Thermal conductivity | 0.033 W/(m·K) (foam, ρ 0.05 g/cm3) |
| Refractive index (nD) | 1.6; dielectric constant 2.6 (1 kHz – 1 GHz) |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Contents
History of Polystyrene
Polystyrene was first found in 1839 by Eduard Simon. He was a pharmacist from Berlin. However, it took until the 1930s for scientists to figure out how to make it in large amounts.
How Polystyrene is Made
Making polystyrene involves a process called polymerization. This is where tiny styrene monomers (single units) are linked together. They form long chains, like beads on a string. This process usually happens by heating the styrene monomers. A special "helper" molecule called an initiator helps them connect.
The way the polymerization process is controlled changes the type of polystyrene that is made. This leads to different kinds of polystyrene with different features.
Types of Polystyrene
There are a few main types of polystyrene, each with different uses:
- General Purpose Polystyrene (GPPS): This is the clear, somewhat brittle type you often see in disposable cups, plates, and food containers. It's light and easy to shape, which makes it popular for packaging. GPPS was first made for sale in the 1930s. Its use quickly grew because it was cheap and could be used for many things. By the 1950s, it was common everywhere.
- Expanded Polystyrene (EPS): This is the white, foamy type you know as packing peanuts. It's also used to insulate buildings and coolers. EPS is made by adding a special gas to the polystyrene during the making process. This gas creates tiny air bubbles inside the plastic. This makes it much lighter and less dense than GPPS. EPS was first made for sale in the 1950s. It became popular quickly because it's great at insulating and is very light.
- High Impact Polystyrene (HIPS): This type of polystyrene is stronger and harder to break than GPPS. It's often used for things like appliance parts and toys. A rubbery material is added during manufacturing to give HIPS its extra strength and flexibility. HIPS was developed after GPPS and EPS, and its use has increased because it lasts longer.
How Polystyrene is Used
Polystyrene can be shaped in different ways. It is often injection molded (melted and pushed into a mold), vacuum formed (heated and sucked over a mold), or extruded (pushed through a shaped opening). Expanded polystyrene is usually extruded or molded in a special way.
Sometimes, polystyrene is mixed with other materials. These are called copolymers. They contain other monomers besides styrene. In recent years, expanded polystyrene has also been mixed with cellulose and starch. Polystyrene is also used in some special polymer-bonded explosives.
Useful Features of Polystyrene
Polystyrene has several features that make it very useful:
- Lightweight: It's much lighter than many other materials. This makes it great for packaging and shipping. It helps save on fuel costs and reduces the environmental impact of moving goods.
- Inexpensive: It's quite cheap to make. This means it can be used for many different products.
- Easy to Mold: It can be easily shaped into many different forms and sizes. This makes it useful for various products.
- Good Insulator: Expanded polystyrene (EPS) is excellent at keeping things hot or cold. This is why it's used in coolers, building insulation, and even some coffee cups.
- Water Resistant: Polystyrene does not soak up water. This makes it good for use in wet places, like disposable food containers.
Environmental Concerns with Polystyrene
Because polystyrene is a plastic, it does not break down easily in nature. This means that polystyrene waste can stay in the environment for many years, even centuries. This is a big problem because it adds to pollution in landfills and oceans.
Animals might not know that polystyrene foam is not natural. They might even mistake it for food. Polystyrene foam is light and floats on water, so it can blow around and end up in rivers and oceans. This can be very harmful to birds or sea animals if they eat large amounts of it.
Interesting Facts About Polystyrene
- Polystyrene does not biodegrade for hundreds of years. It is also hard for sunlight to break it down.
- Whether polystyrene containers can be used safely in a microwave with food is a debated topic. Some containers are safe, but only if they are clearly labeled for microwave use.
- Like other chemical compounds, polystyrene can catch fire. Foamed polystyrene materials have accidentally caused large fires and loss of life.
- In 1988, Suffolk County, New York, was the first place in the U.S. to ban polystyrene in general.
- In 2015, scientists found that mealworms could eat and live healthily on a diet of expanded polystyrene. About 100 mealworms could eat 34 to 39 milligrams of this foam in a day. The mealworms' droppings were found to be safe for use as soil for plants.
- In March 2022, scientists Sewon Oh and Erin Stache at Cornell University found a new way to recycle polystyrene. They could turn it into benzoic acid. This process involved shining light on polystyrene with iron chloride and acetone for 20 hours.
Images for kids
-
Polystyrene is flammable and makes a lot of black smoke when it burns.
See also
 In Spanish: Poliestireno para niños
In Spanish: Poliestireno para niños







