Propene facts for kids
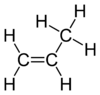
Propene is a special type of chemical compound that scientists call an organic compound. It's also known as propylene, and its chemical formula is C3H6. It's the second simplest chemical in a group called alkenes. Because it's only made of hydrogen and carbon atoms, we call it a hydrocarbon. At normal room temperature and pressure, propene is a gas.
Quick facts for kids Propene |
|
|---|---|
| IUPAC name | Propene |
| Identifiers | |
| CAS number | |
| PubChem | |
| KEGG | C11505 |
| ChEBI | CHEBI:16052 |
| RTECS number | UC6740000 |
| SMILES | C=CC |
|
InChI
InChI=1/C3H6/c1-3-2/h3H,1H2,2H3
|
|
| Properties | |
| Molecular formula | |
| Molar mass | 0 g mol-1 |
| Appearance | Colorless gas |
| Density | 1.81 kg/m3, gas (1.013 bar, 15 °C) 613.9 kg/m3, liquid |
| Melting point | |
| Boiling point | |
| 0.61 g/m3 | |
| Viscosity | 8.34 µPa·s at 16.7 °C |
| Structure | |
| Dipole moment | 0.366 D (gas) |
| Hazards | |
| MSDS | External MSDS |
| Main hazards | Highly flammable, Asphyxiant |
| NFPA 704 |
|
| R-phrases | 12 |
| S-phrases | 9-16-33 |
| Flash point | −108 °C |
| Related compounds | |
| Related groups | Allyl, Propenyl |
| Related compounds | Propane, Propyne Propadiene, 1-Propanol 2-Propanol |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
What Propene is Used For
Propene is made from things like fossil fuels and coal. It's super important in the petrochemical industry (which makes chemicals from oil and gas), second only to ethene.
Making Plastics and Other Chemicals
About two-thirds of all propene is used to make polypropylene, a common plastic. This plastic is used in many everyday items, from car parts to food containers.
Propene and benzene are also combined to create other useful chemicals. These include acetone (found in nail polish remover) and phenol (used in many products like plastics and medicines).
Propene is also used to make:
- Isopropanol (also known as rubbing alcohol)
- Acrylonitrile (used to make acrylic fibers and plastics)
- Propylene oxide (used to make plastics and other chemicals)
- Epichlorohydrin (used to make epoxy resins)
Images for kids
See also
 In Spanish: Propileno para niños
In Spanish: Propileno para niños 
 | Charles R. Drew |
 | Benjamin Banneker |
 | Jane C. Wright |
 | Roger Arliner Young |