Hydrogen chloride facts for kids
Quick facts for kids Hydrogen chloride |
|
|---|---|
| IUPAC name | Hydrogen chloride |
|
Chlorane
|
|
| Other names | Hydrochloric acid gas Hydrochloric gas Hydrochloride |
| Identifiers | |
| CAS number | |
| PubChem | |
| EC number | 231-595-7 |
| KEGG | D02057 |
| MeSH | |
| ChEBI | CHEBI:17883 |
| RTECS number | MW4025000 |
| SMILES | Cl |
|
InChI
InChI=1/HCl/h1H
|
|
| Beilstein Reference | 1098214 |
| Gmelin Reference | 322 |
| Properties | |
| Molecular formula | |
| Molar mass | 0 g mol-1 |
| Appearance | Colorless gas |
| Odor | pungent; sharp and burning |
| Density | 1.49 g/L |
| Melting point | |
| Boiling point | |
| 823 g/L (0 °C) 720 g/L (20 °C) 561 g/L (60 °C) |
|
| Solubility | soluble in methanol, ethanol, ether and water |
| Vapor pressure | 4352 kPa (at 21.1 °C) |
| Acidity (pKa) | −3.0; −5.9 (±0.4) |
| Basicity (pKb) | 17.0 |
| Conjugate acid | Chloronium |
| Conjugate base | Chloride |
| Refractive index (nD) | 1.0004456 (gas) 1.254 (liquid) |
| Viscosity | 0.311 cP (−100 °C) |
| Structure | |
| Molecular shape | linear |
| Dipole moment | 1.05 D |
| Thermochemistry | |
| Std enthalpy of formation ΔfH |
−92.31 kJ/mol |
| Std enthalpy of combustion ΔcH |
−95.31 kJ/mol |
| Standard molar entropy S |
186.902 J/(K·mol) |
| Specific heat capacity, C | 0.7981 J/(K·g) |
| Pharmacology | |
| ATC code | |
| Hazards | |
| Main hazards | Toxic, corrosive |
| NFPA 704 |
|
| U.S. Permissible exposure limit (PEL) |
C 5 ppm (7 mg/m3) |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Hydrogen chloride is a special chemical compound. Its chemical formula is HCl. It belongs to a group called hydrogen halides. At normal room temperature, it's a clear, colorless gas. When this gas touches the moisture in the air, it creates white fumes. These fumes are actually tiny droplets of hydrochloric acid. Both hydrogen chloride gas and hydrochloric acid are very important in many industries and technologies. Hydrochloric acid is simply hydrogen chloride dissolved in water.
Contents
Understanding Hydrogen Chloride
Hydrogen chloride is a diatomic molecule. This means it's made of two atoms. One is a hydrogen atom (H) and the other is a chlorine atom (Cl). These two atoms are joined by a strong connection called a polar covalent bond.
A Polar Molecule
The chlorine atom pulls electrons much more strongly than the hydrogen atom. This makes the bond "polar." Think of it like a magnet with two ends. One end of the molecule (the chlorine side) has a slightly negative charge. The other end (the hydrogen side) has a slightly positive charge. This "polarity" helps HCl dissolve easily in water.
How it Becomes an Acid
When hydrogen chloride gas touches water (H
2O), they react. They form hydronium ions ([H
3O]+
) and chloride ions (Cl−
). This reaction makes a solution called hydrochloric acid.
- HCl + H
2O → [H
3O]+
+ Cl−
Hydrochloric acid is known as a strong acid. This means that almost all the HCl molecules break apart in water. They form ions, making the solution very acidic. Even without water, hydrogen chloride can act as an acid. For example, it can dissolve in other liquids like methanol.
- HCl + CH
3OH → [CH
3OH
2]+
+ Cl−
Because it's an acid, hydrogen chloride can be corrosive. This is especially true when moisture is present.
What Hydrogen Chloride Looks Like
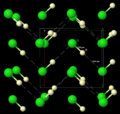
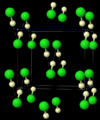
Hydrogen chloride is a colorless gas. It has a strong, sharp, and burning smell. When it gets very cold, it turns into a solid. This solid form has a special structure where the molecules link together in zigzag chains.
| Temperature (°C) | 0 | 20 | 30 | 50 |
|---|---|---|---|---|
| Water | 823 | 720 | 673 | 596 |
| Methanol | 513 | 470 | 430 | |
| Ethanol | 454 | 410 | 381 | |
| Ether | 356 | 249 | 195 |
This table shows how much hydrogen chloride can dissolve in different liquids at various temperatures. You can see it dissolves a lot in water!
How Hydrogen Chloride is Made
Most of the hydrogen chloride made today is used to produce hydrochloric acid.
Making it from Basic Elements
One way to make hydrogen chloride is by directly combining two gases: chlorine gas (Cl
2) and hydrogen gas (H
2).
- Cl
2 + H
2 → 2 HCl
This reaction creates heat, so the equipment used is often called an HCl "oven" or "burner." The hydrogen chloride gas produced is then absorbed into very pure water. This creates super clean hydrochloric acid, often used in the food industry. Blue light can also start this reaction.
Making it During Other Chemical Processes
Hydrogen chloride is often made as a side product when creating other important chemicals. These include plastics like Teflon and PVC. It's also made when creating CFCs (though many CFCs are now banned due to environmental concerns). In these processes, hydrogen atoms in organic compounds are replaced by chlorine atoms. The leftover hydrogen then combines with chlorine to form hydrogen chloride.
- RH + Cl
2 → RCl + HCl
This hydrogen chloride can be used again or dissolved in water to make industrial-grade hydrochloric acid.
Making Small Amounts in the Lab
For school or lab experiments, small amounts of hydrogen chloride can be made. One way is to take hydrochloric acid and remove its water using sulfuric acid or calcium chloride. Another common method is reacting sulfuric acid with sodium chloride (table salt):
- NaCl + H
2SO
4 → NaHSO
4 + HCl↑
This reaction happens at room temperature. If you heat it above 200 °C and there's still salt, another reaction can occur:
- NaCl + NaHSO
4 → Na
2SO
4 + HCl↑
It's important that the chemicals used in these lab methods are dry. Hydrogen chloride can also be made by adding cold water slowly to certain reactive chlorine compounds, like phosphorus pentachloride (PCl
5).
- PCl
5 + H
2O → POCl
3 + 2 HCl↑
Everyday Uses of Hydrogen Chloride
Most hydrogen chloride is used to make hydrochloric acid. It's also a key ingredient in making vinyl chloride. Vinyl chloride is the main building block for PVC, a plastic used in pipes, window frames, and many other products. Hydrogen chloride is also used to create trichlorosilane. This chemical is important for making very pure silicon, which is used in computer chips and solar panels.
- Si + 3 HCl → HSiCl
3 + H
2
A Look Back: History of HCl
People have known about hydrogen chloride and hydrochloric acid for a very long time. Around the year 900, scientists like Jabir ibn Hayyan and Abu Bakr al-Razi were experimenting with sal ammoniac (a type of salt). They found that mixing it with other chemicals could produce hydrogen chloride gas. At first, they didn't realize how useful this gas was.
A big step forward was the discovery of aqua regia (royal water) around the 1300s. This powerful liquid could dissolve gold! It was made by adding sal ammoniac to nitric acid. This showed how important chemicals derived from hydrogen chloride could be.
Later, in the 17th century, Johann Rudolf Glauber used salt and sulfuric acid to make sodium sulfate. This process also released hydrogen chloride gas. In 1772, Joseph Priestley successfully prepared pure hydrogen chloride. By 1810, Humphry Davy proved that it was made of hydrogen and chlorine.
During the Industrial Revolution, there was a huge need for chemicals like soda ash. Nicolas Leblanc developed a way to make soda ash that produced hydrogen chloride as a byproduct. Initially, this gas was just released into the air. However, laws like the Alkali Act 1863 in Britain stopped this pollution. So, factories started dissolving the waste HCl gas in water, which led to the large-scale production of hydrochloric acid.
In the 20th century, hydrogen chloride was used to make important plastics. These included chloroprene (used in Neoprene rubber) and vinyl chloride (used in PVC). For example, to make vinyl chloride, HCl was added to acetylene (C
2H
2).
Staying Safe with Hydrogen Chloride
Hydrogen chloride is a dangerous chemical. When it touches water in your body, it forms corrosive hydrochloric acid.
- Breathing it in: Inhaling the fumes can cause coughing, choking, and irritation in your nose, throat, and lungs. In serious cases, it can harm your lungs and even be deadly.
- Skin contact: If it touches your skin, it can cause redness, pain, and severe chemical burns.
- Eye contact: Getting hydrogen chloride in your eyes can cause severe burns and permanent eye damage.
Always be very careful around this chemical and follow all safety rules.
Images for kids
See also
 In Spanish: Cloruro de hidrógeno para niños
In Spanish: Cloruro de hidrógeno para niños
- Gastric acid, hydrochloric acid secreted into the stomach to aid digestion of proteins
- Chloride, salts of hydrogen chloride
- Hydrogen bromide
- Hydrochloride, organic salts of hydrochloric acid
- Hydrochlorination, addition reaction with alkenes
- Hydrochloric acid
- Hydrogen
- Chlorine
 | Frances Mary Albrier |
 | Whitney Young |
 | Muhammad Ali |