Ammonium chloride facts for kids
Quick facts for kids Ammonium chloride |
|
|---|---|
 |
|
 |
|
| IUPAC name | Ammonium chloride |
| Other names | Sal ammoniac, Salmiac, Nushadir salt, Sal armagnac, Salt armoniack, Salmiak |
| Properties | |
| Molecular formula | |
| Molar mass | 0 g mol-1 |
| Appearance | White solid, hygroscopic |
| Odor | Odorless |
| Density | 1.519 g/cm3 |
| Melting point | |
| Boiling point | |
| 383.0 g/L (25 °C) | |
| Solubility | Soluble in liquid ammonia, hydrazine, Slightly soluble in acetone Insoluble in diethyl ether, ethyl acetate |
| Solubility in methanol | 35.4 g/kg (25 °C) |
| Solubility in ethanol | 6 g/L (19 °C) |
| Related compounds | |
| Other anions | Ammonium fluoride Ammonium bromide Ammonium iodide |
| Other cations | Sodium chloride Potassium chloride Hydroxylammonium chloride |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Ammonium chloride is a common chemical compound. It is made of two parts: ammonium and chloride ions. It looks like a white, clear crystal. You might know it by its other names, like "sal ammoniac" or "salmiac."
This compound is used in many different ways. It helps join metals together and powers some types of batteries. It can also change into a gas when heated.
Contents
What is Ammonium Chloride?
Ammonium chloride is a type of salt. It is not the same as table salt, which is sodium chloride. It forms when ammonia (a gas) and hydrochloric acid (a strong acid) mix.
Its Chemical Makeup
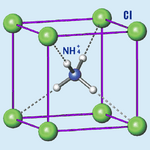
Ammonium chloride has the chemical formula NH4Cl. This means it has one nitrogen atom, four hydrogen atoms, and one chlorine atom. It is a very stable compound.
How is Ammonium Chloride Used?
Ammonium chloride has many practical uses in daily life and industry.
In Soldering and Metalwork
One of its main uses is in soldering. When you solder, you join metal pieces together. Ammonium chloride helps clean the metal surfaces. It removes rust and other unwanted layers. This makes it easier for the solder to stick.
In Batteries
Ammonium chloride is also used in some types of batteries. It acts as an electrolyte. An electrolyte is a substance that helps electricity flow. It was a key part of the older Leclanche cell. This was one of the first successful types of batteries.
Other Uses
- Medicine: Sometimes it is used in cough medicines. It can help clear mucus from the lungs.
- Food: In some countries, it is used as a food additive. It gives a salty, slightly sour taste to licorice candy.
- Fertilizers: Farmers use it as a source of nitrogen for plants. Nitrogen is important for plant growth.
Properties of Ammonium Chloride
Ammonium chloride is a white solid. It does not have a smell. It dissolves easily in water. When it dissolves, it makes the water slightly acidic.
Heating and Cooling
When you heat ammonium chloride, it does something special. It turns directly into a gas without becoming a liquid first. This process is called sublimation. If you cool the gas, it turns back into a solid. This is why it is called a "sublime compound."
Safety Information
Ammonium chloride is generally safe when used correctly. However, like many chemicals, it should be handled with care. If you swallow too much, it can be harmful. It can also irritate your eyes if it gets into them. Always follow safety rules when working with chemicals.
Related pages
Images for kids
See also
 In Spanish: Cloruro de amonio para niños
In Spanish: Cloruro de amonio para niños
 | Shirley Ann Jackson |
 | Garett Morgan |
 | J. Ernest Wilkins Jr. |
 | Elijah McCoy |



