Battery facts for kids

Different types of batteries and cells: two AA, one D, one ham radio battery, two 9-volt (PP3), two AAA, one C, one camcorder battery, one cordless phone battery
|
|
| Type | Power source |
|---|---|
| Working principle | Chemical reactions that create electricity |
| First production | 1800s |
| Electronic symbol | |
 The symbol for a battery in a circuit diagram. It looks like the first battery ever made, a voltaic pile. |
|
A battery is a device that can store electricity. Some batteries can be used again and again (rechargeable), while others are used once and then thrown away. Batteries store a type of electricity called direct current (DC).
Often, when people say "battery," they are actually talking about a single "cell." A true battery is usually made of two or more cells connected together. For example, AA, AAA, C, and D cells all give out 1.5 volts of power. The amount of power (voltage) a cell provides depends on the chemicals inside it. How much energy it can store, or how long it can last, depends on its size. A bigger cell can usually provide more power or last longer.
The chemical reactions inside a battery create heat. This is why a laptop battery might feel warm after being used for a while. Some batteries, like the lithium ones in laptops, can even be a fire risk, though this is very rare.
Batteries come in many shapes, sizes, and power levels (voltages). It's possible to use different sized batteries for special projects, but it can be tricky. Batteries are usually more expensive than getting power from a wall outlet (mains electricity). However, they are perfect for things that need to move around, like phones or flashlights.
Some bicycles have tail-lights that run on batteries, or sometimes on a small generator powered by the wheels. You can also find hand-crank or wind-up generators that can power small radios or flashlights, which means you don't need batteries for them.
Rechargeable batteries work by reversing the chemical reactions inside them when you plug them into a charger. But even rechargeable batteries can only be recharged a certain number of times. Each time you recharge one, it might hold a little less power. It's very important never to try and recharge a battery that isn't meant to be recharged, as the chemicals inside could leak out.
Contents
History of Batteries
The idea of storing electricity might go back to the Middle East around 1000 B.C., but the first modern battery was invented much later.
The first true battery was created in 1800 by Alessandro Volta. Today, we call his invention the voltaic pile.
Older batteries used to be glass bottles filled with a liquid and metal rods. People had to be careful not to tip them over, or the liquid would spill.
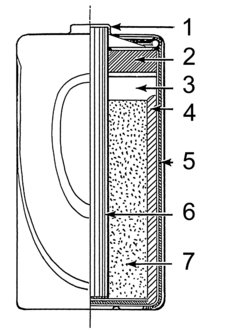
Modern batteries use a special paste instead of a liquid, which is soaked up inside. Everything is sealed tightly in a case, so nothing can spill out.
Battery Sizes and Types
Batteries come in many different shapes, sizes, and power levels (voltages).
Common sizes like AA, AAA, C, and D cells, including alkaline batteries, are standard. They usually provide about 1.5 volts. The voltage depends on the chemicals used. How much electric charge a battery can supply depends on its size and the chemicals inside. This charge is often measured in "ampere-hours." Since the voltage stays the same, a bigger cell can provide more power (amps) or last for a longer time.
Primary and Secondary Batteries
Batteries are divided into two main types:
- Primary batteries are designed to be used once until they run out of energy, and then you throw them away. Their chemical reactions usually can't be reversed, so they can't be recharged. Once the chemicals are used up, the battery stops working.
- Secondary batteries can be recharged. You can apply electric current to them, which reverses the chemical reactions. This brings the original chemicals back, so the battery can be used, recharged, and used again many times.
Primary batteries, like the ones you use in a TV remote, start working right away. They are best for devices that don't use much power, are used only sometimes, or are far from an electrical outlet. Common types include zinc–carbon and alkaline batteries.
Secondary batteries, also called rechargeable batteries, usually need to be charged before you use them for the first time. The oldest type of rechargeable battery is the lead–acid battery, which is widely used in cars and boating. These batteries are heavy but can provide a lot of power quickly, which is why they are great for starting car engines.
How Batteries are Made
Many different types of batteries have been made, each with unique chemical processes and designs.
A wet cell battery has a liquid inside called an electrolyte. These batteries were common before dry cells. They are still used in car batteries and for backup power in industries.
A dry cell uses a paste instead of a liquid, with just enough moisture for electricity to flow. Because there's no free liquid, a dry cell can be used in any position without spilling. This makes them perfect for portable devices. A common dry cell is the zinc–carbon battery, which gives about 1.5 volts.
A reserve battery can be stored for a very long time, sometimes years, without being used. When you need it, you activate it (for example, by adding a liquid electrolyte), and then it's ready to work. These are often used in special situations where a battery needs to be ready instantly after long storage, like in some military devices.
Scientists are always working on new types of batteries. For example, a new type of solid-state battery is being developed that could be safer, charge faster, and last longer for phones, electric cars, and storing energy. These new batteries might also use more eco-friendly materials like sodium from seawater.
There are also biological batteries that create electricity from sugar, similar to how living things produce energy.
Modern rechargeable batteries for phones and laptops include types like nickel-cadmium (NiCd), nickel metal hydride (NiMH), and lithium-ion (Li-ion) cells. Lithium-ion batteries are the most popular for rechargeable devices today because they store a lot of energy for their size.
Newer developments include batteries with built-in electronics, like USBCELLs that can be charged with a USB cable, and "smart" battery packs that protect the battery from damage.
Consumer and Industrial Batteries
Batteries are made for both everyday users (consumers) and for industrial uses. Industrial batteries are often more expensive because they are built to be stronger, last longer, and work better in tough conditions like high heat or humidity.
How Batteries are Used Together
Standard batteries fit into a battery holder in a device. But for devices that don't use standard sizes, batteries are often put together into a special battery pack. These packs can also have a battery management system to make sure the batteries charge and discharge evenly and safely.
Battery Lifespan
The "life" of a battery can mean two things. For rechargeable batteries, it can mean how long a device runs on a full charge, or how many times the battery can be charged and discharged before it stops working well. For non-rechargeable batteries, it just means how long it lasts until it's used up. Shelf life is how long a battery keeps its power when it's just sitting unused.
All batteries lose some power when it's cold. The Zamboni pile, invented in 1812, is famous for lasting a very long time without needing to be recharged, though it only produces a tiny amount of electricity. The Oxford Electric Bell has been ringing almost non-stop since 1840 using its original Zamboni piles!
Disposable batteries typically lose 8–20% of their power each year if stored at room temperature. This is called "self-discharge." Storing batteries in a cooler place, like a refrigerator, can slow this down, but some batteries can be damaged by freezing. Older rechargeable batteries, especially nickel-based ones, self-discharge faster than disposable ones. However, newer low self-discharge NiMH batteries and modern lithium batteries lose power much slower.
Every time a battery is charged and discharged, the materials inside change a little. This can cause the battery to lose some capacity over time. For example, low-capacity NiMH batteries can be charged about 1,000 times, while high-capacity ones last about 500 cycles. Charging a battery too quickly can also shorten its life. If a charger doesn't know when a battery is full, it might overcharge it, which can cause damage.
Some older nickel-based batteries could develop a "memory effect" if used in a certain way, making them seem to lose capacity. This is less of a problem with newer batteries.
Car batteries are designed to handle vibrations, shocks, and different temperatures. Because of these stresses, most car batteries don't last more than six years. Car batteries are usually only slightly discharged before they are recharged. "Deep-cycle" batteries, like those in electric golf carts, have thicker parts and are designed to be discharged more deeply.
You can make batteries last longer by storing them in a cool place, like a refrigerator or freezer, which slows down the chemical reactions that cause them to lose power. However, some battery makers don't recommend this.
Battery Dangers
A battery explosion usually happens because it's used incorrectly or breaks down, like trying to recharge a battery that isn't rechargeable, or if there's a short circuit.
If a battery is recharged too fast, it can produce explosive gases like hydrogen and oxygen faster than they can escape. This builds up pressure, causing the battery case to burst. In serious cases, battery chemicals can spray out and cause injury. For example, if a lithium-ion battery is charged too quickly, it can short circuit and lead to fires or explosions. Car batteries can explode if a short circuit creates a lot of current, producing a lot of hydrogen gas that can be ignited by a spark.
Overcharging a battery (trying to put more electricity into it than it can hold) can also cause it to explode, leak, or be permanently damaged. It can also harm the charger or the device the battery is used in.
Throwing a battery into a fire can also cause it to explode as steam builds up inside the sealed case.
Many battery chemicals are harmful if they leak out. If a battery leaks, the chemicals can damage or ruin the device it's in. That's why many electronics makers suggest taking batteries out of devices you won't use for a long time.
Many types of batteries contain toxic materials like lead, mercury, and cadmium. When batteries are old, they must be disposed of properly to protect the environment. Batteries are a type of electronic waste (e-waste). Recycling services can recover these toxic substances, which can then be used to make new batteries.
Batteries can be dangerous if swallowed. Small button cells are especially risky for young children. If swallowed, the battery's electrical current can cause serious tissue damage in the digestive system, which can sometimes be fatal. Some battery makers have added a bad taste to batteries to discourage children from swallowing them.
Future of Batteries
The demand for batteries has been growing very quickly, especially for electric cars and for storing energy in power grids. This growth is happening because people want to move away from burning fossil fuels and use cleaner, renewable energy sources.
Electric batteries in things like battery electric vehicles and home energy storage systems are becoming important parts of "smart grids." These smart grids can use batteries to store energy when it's cheap (like from solar panels during the day) and release it when it's needed later.
New ways of reusing batteries, like giving old electric car batteries a "second life" for backup power or renewable energy storage, are also becoming popular. This helps reduce waste and makes batteries even more useful.
Large-scale battery storage systems are being built to collect and store energy from power plants or the grid. They can then release this energy later to provide electricity or other services when needed, making our power supply more reliable.
Comparing Battery Types
Different battery types have different features, like how much power they give, how much energy they store for their size, if they can catch fire, how they are built, what temperatures they can work in, and how long they last on the shelf. These features are decided by the chemicals inside the battery.
| Chemistry | Nominal voltage (V) | Specific energy (energy per weight) | Notes | Shelf life at 25 °C (months) |
|---|---|---|---|---|
| Zinc–carbon | 1.2 | 130 kJ/kg | Inexpensive. | 18 |
| Alkaline (zinc–manganese dioxide) | 1.15 | 400-590 kJ/kg | Good for many uses. | 30 |
| Lithium (lithium–iron disulfide) | 1.5 | 1070 kJ/kg | More expensive. Used in 'plus' or 'extra' batteries. | 337 |
| Lithium (lithium–manganese dioxide) | 3.0 | 830–1010 kJ/kg | Expensive. Used for high-power devices or long storage. | |
| Zinc–air | 1.1 | 1590 kJ/kg | Mostly used in hearing aids. | |
| Zamboni pile | 0.8 | Very long life, but very low power. | >2,000 | |
| Silver oxide (silver–zinc) | 1.5 | 470 kJ/kg | Very expensive. Used in small 'button' cells. | 30 |
| Chemistry | Cell voltage | Specific energy (energy per weight) | Notes |
|---|---|---|---|
| NiCd | 1.2 | 140 kJ/kg | Inexpensive. Can handle high power use. Contains Cadmium, which is harmful to the environment. |
| Lead–acid | 2.1 | 140 kJ/kg | Moderately expensive. Used in car batteries. Contains Lead, which is harmful. |
| NiMH | 1.2 | 360 kJ/kg | Inexpensive. Better than alkaline for high-power devices. Used in some cars. |
| NiZn | 1.6 | 360 kJ/kg | Moderately inexpensive. Good for high-power devices. No toxic parts. |
| Lithium ion | 3.6 | 460 kJ/kg | Very expensive. Very high energy for its size. Common in laptops, cameras, phones. Can be dangerous if not made or used carefully. |