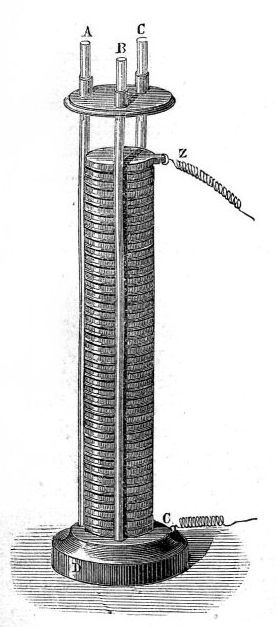
Voltaic pile facts for kids
The voltaic pile was the very first electric battery! It was created in the year 1800 by Alessandro Volta, a smart scientist from Italy.
Contents
How the Voltaic Pile is Built
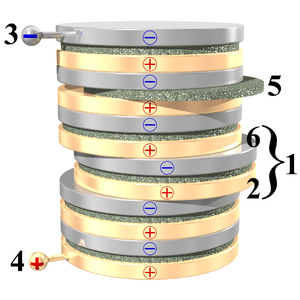
Look at the picture on the right. A voltaic pile is a stack of round discs. These discs are made from three different materials. Some discs are made of copper, and others are made of zinc. You can use other metals too, but it's important to use two different kinds of metal.
There are also discs made from leather, fabric, or cardboard. These are the green discs in the picture. They must be soaked in something acidic, like vinegar or lemon juice.
Notice how the discs are stacked in a pattern. First, there's a disc of one metal, then a soaked cardboard or leather disc, then a disc of the other metal. This pattern repeats over and over.
In the picture, one "set" of discs (zinc, cardboard, copper) is pushed a little to the right. This "set" is called a cell. The voltaic pile in the picture has 6 cells. Each cell creates a small amount of electricity, about 1.1 volts. When you stack many cells, their voltages add up. So, the pile in the picture gives 6 × 1.1 volts, which is 6.6 volts!
How the Voltaic Pile Works
The acid in the leather, fabric, or cardboard discs tries to dissolve some of the metal in the zinc discs. When a zinc atom dissolves, it gives away two of its electrons. The zinc atom then becomes an ion. Ions are atoms that have lost or gained electrons.
These zinc ions can move freely through the liquid acid. They travel through the soaked disc and reach the copper disc. Since the zinc ions are "missing" their two electrons, they "steal" two electrons from the copper disc. When they "steal" electrons, the zinc ions turn back into "normal" zinc atoms. You can also use saltwater instead of acid in the soaked discs.
So, here's what happens: the zinc disc ends up with many electrons left behind by the zinc ions. The copper disc ends up "missing" many electrons that were "stolen" by the zinc ions. To use the electricity, you connect something that needs power to the discs at the top and bottom of the pile. This connection allows the extra electrons from the zinc disc to flow to the copper disc. At the copper disc, these electrons replace the ones that were "stolen" by the zinc. This flow of electrons is electricity!
How Volta Got the Idea
Luigi Galvani was an Italian scientist who studied anatomy. Around 1786, he saw something amazing. He had cut apart a frog. When he touched one of its legs with two tools made from different metals, the leg moved! This happened even though the frog was already dead.
Galvani thought the movement came from something special in the frog's leg. But Volta realized that the tools had to be made from two different metals for the leg to move. Volta also found that he could make a little electricity just by touching two different metals together. He called this "contact tension." Galvani and Volta discussed this a lot, but they never agreed on why it happened.
Volta decided to experiment more. He tried different metal rods in wine bottles. He discovered that silver and zinc worked best together. He also found that using flat discs instead of rods made the electricity stronger. This is because discs have more surface area. More surface area means more "room" for the zinc ions to leave one disc and connect with the other.
Originally, Volta added an extra disc of each metal at the very top and bottom of his stack. Later, scientists found out these extra discs weren't needed.
What Happened After the Invention
Before the voltaic pile, people could only make static electricity. This is the kind of electricity you get when you rub a balloon on your hair. But with the voltaic pile, scientists could make electricity that kept flowing for a long time!
The voltaic pile was also easy to build. This new kind of steady electricity allowed scientists to do many "new" things. For example, when new kinds of metal were discovered, the electricity from a voltaic pile could "sort" chemicals. This helped create small, very pure pieces of these "new" metals. It was a huge step forward in understanding and using electricity!
Images for kids
-
Drawing of the voltaic pile in different setups, from the letter sent by Alessandro Volta to Joseph Banks.
 | Bessie Coleman |
 | Spann Watson |
 | Jill E. Brown |
 | Sherman W. White |