Alessandro Volta facts for kids
Quick facts for kids
Alessandro Volta
|
|
|---|---|
 |
|
| Born | 18 February 1745 |
| Died | 5 March 1827 (aged 82) Como, Lombardy-Venetia, Austrian Empire
|
| Nationality | Italian |
| Known for |
|
| Awards |
|
| Scientific career | |
| Fields | Physics and chemistry |
Alessandro Volta was an Italian scientist born in 1745. He was a pioneer in studying electricity. He is famous for inventing the electric battery and discovering methane gas.
In 1799, he invented the voltaic pile, which was the first electric battery. He shared his findings with the Royal Society in 1800. Volta's invention showed that electricity could be made using chemicals. This was a big deal because many people thought electricity only came from living things.
His discovery excited many scientists. It led to the study of electrochemistry, which is about how electricity and chemical reactions are linked. Even Napoleon Bonaparte admired Volta's invention. He invited Volta to show his work at the Institute of France. Volta received many honors from Napoleon throughout his life.
Volta taught experimental physics at the University of Pavia for almost 40 years. His students looked up to him greatly. Even though he was very successful, Volta preferred a quiet family life. In his later years, he lived away from public view. He passed away in 1827 after several years of illness. The unit of electric potential is named the volt in his honor.
Contents
Volta's Early Life and Discoveries
Volta was born in Como, a town in northern Italy, on February 18, 1745. In 1794, he married Teresa Peregrini, who was also from Como. They had three sons: Zanino, Flaminio, and Luigi. His father, Filippo Volta, came from a noble family. His mother, Donna Maddalena, was from the Inzaghi family.
In 1774, Volta became a physics professor at the Royal School in Como. A year later, he improved a device called the electrophorus. This machine could produce static electricity. He made it so popular that many people thought he invented it. However, a similar machine was described earlier by a Swedish scientist named Johan Wilcke. In 1777, Volta traveled through Switzerland and became friends with Horace Bénédict de Saussure.
Between 1776 and 1778, Volta studied the chemistry of gases. He discovered methane after reading about "flammable air" from Benjamin Franklin. In November 1776, he found methane in the marshes near Angera. By 1778, he was able to get pure methane. He even did experiments like lighting methane with an electric spark in a closed container.
Volta also studied electrical capacitance. This is about how much electric charge an object can store. He found that for a given object, the electric charge (Q) is directly related to the electric potential difference (V). This is known as Volta's Law of Capacitance. Because of this work, the unit of electrical potential is called the volt.
In 1779, he became a professor of experimental physics at the University of Pavia. He held this important position for nearly 40 years. Volta's classes were so popular that a new "physical theater" was built for his lectures. This building is now called the "Aula Volta." The emperor also gave Volta money to buy scientific tools from England and France. Today, about 150 of these tools are displayed at the University History Museum in Pavia.
Volta and Galvani's Discovery
Luigi Galvani, another Italian physicist, found something he called "animal electricity." He noticed that a frog's leg would twitch when touched by two different metals connected together. Volta realized that the frog's leg acted like a conductor (what we now call an electrolyte) and an electricity detector.
He understood that the frog's leg itself was not making the electricity. The electricity came from the two different metals. So, he replaced the frog's leg with paper soaked in salt water. He then used other methods to detect the flow of electricity.
This led him to discover the electrochemical series. This series shows how different metals react with each other to produce electricity. He found that the electromotive force (emf) of a galvanic cell (a simple battery) depends on the difference between the electrical potentials of the two metals used.
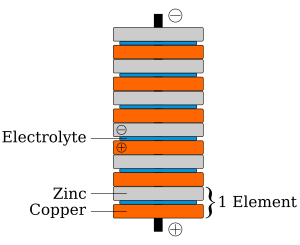
In 1800, after a disagreement with Galvani about "animal electricity," Volta invented the voltaic pile. This was an early electric battery that could produce a steady electric current. Volta found that zinc and copper were the best metals to use together. He first experimented with individual cells, each being a wine goblet filled with salt water with the two metals dipped in. The voltaic pile was an improvement, using layers of zinc, copper, and cardboard soaked in salt water.
How Volta's Early Battery Worked
When Volta announced his invention of the voltaic pile, he gave credit to other scientists like William Nicholson, Tiberius Cavallo, and Abraham Bennet.
Volta's battery is considered one of the first electrochemical cells. It had two parts called electrodes: one made of zinc and the other of copper. The liquid part, called the electrolyte, was either sulfuric acid mixed with water or salt water.
Here's a simple way it worked:
- The zinc metal reacted with the acid or salt water.
- This reaction created electrons.
- These electrons moved to the copper electrode.
- This made the zinc rod the negative end and the copper rod the positive end of the battery.
- When you connected the two ends, an electric current would flow.
However, this type of battery had some problems. It was not very safe to use because sulfuric acid can be dangerous. Also, the battery's power would decrease over time. This happened because hydrogen gas would build up on the copper electrode, stopping the reaction.
Later Years and Legacy
In 1809, Volta became a member of the Royal Netherlands Academy of Arts and Sciences. In 1810, Napoleon Bonaparte made him a count to honor his work.
Volta retired in 1819 to his home in Camnago, a small town near Como, Italy. This town is now called "Camnago Volta" in his honor. He passed away there on March 5, 1827, shortly after his 82nd birthday. Volta's body was buried in Camnago Volta.
Volta's Lasting Impact
Volta's achievements are remembered at the Tempio Voltiano memorial in Como. This museum displays some of the equipment Volta used for his experiments. Nearby is the Villa Olmo, home to the Voltian Foundation, which supports scientific activities. Volta did much of his important research and made his first inventions near Como.
At the Old Campus of the University of Pavia, you can see the "Aula Volta" classroom. Emperor Joseph II ordered this room built in 1787 for Volta's lectures. The University History Museum also has many of Volta's scientific instruments, his chair, and his blackboard.
Volta's image was even on the Italian 10,000 Lire note from 1990 to 1997. The note also showed a drawing of his voltaic pile.
In late 2017, a new computer chip design for powerful graphics cards was named Volta by Nvidia.
In 2019, a new species of electric eel, Electrophorus voltai, was discovered. It is the strongest electricity producer in nature and was named after Volta.
Images for kids
-
Volta explains the principle of the "electric column" to Napoleon in 1801
See also
 In Spanish: Alessandro Volta para niños
In Spanish: Alessandro Volta para niños
- Armstrong effect
- Eudiometer
- History of the battery
- History of the internal combustion engine
- Lemon battery
- Mercury beating heart
- Thermoelectric effect
- Volta pistol
- Volta potential
- Volta (lunar crater)
- Volta Prize
 | Janet Taylor Pickett |
 | Synthia Saint James |
 | Howardena Pindell |
 | Faith Ringgold |