Electrochemistry facts for kids
Electrochemistry is a special part of chemistry. It looks at how chemical reactions can create electricity, and how electricity can make chemical reactions happen. Think of it like a bridge between chemistry and electricity!
These cool reactions usually happen at special spots called electrodes. Electrodes are made of materials that can conduct electricity, like metals. They are placed in a liquid called an electrolyte. An electrolyte is a solution that has tiny charged particles called ions floating in it. These ions help carry the electric current.
Electrochemistry became a real science when the first electric battery, called the voltaic pile, was invented. This invention showed how chemical reactions could make a steady flow of electricity.
Contents
What is Electrochemistry?
Electrochemistry studies how electrons move during chemical reactions. When electrons move, they create an electric current. This is how batteries work! In some reactions, electricity is used to force a chemical change that wouldn't happen on its own. This is called electrolysis.
How Does Electrochemistry Work?
Imagine two electrodes dipped into an electrolyte. One electrode is where electrons are given away by atoms or molecules. This is called oxidation. The other electrode is where electrons are gained. This is called reduction. Together, these two parts make up a redox reaction.
- Electrodes: These are the parts where the chemical reactions happen. They can be made of different metals or carbon.
- Electrolyte: This is the liquid or paste that allows ions to move between the electrodes. It completes the electrical circuit.
- Electron Flow: Electrons move from one electrode to the other through an outside wire. This movement is what we call electricity.
Where Do We See Electrochemistry?
Electrochemistry is all around us! It powers many things we use every day.
Batteries and Fuel Cells
Batteries are probably the most common example of electrochemistry. They turn chemical energy into electrical energy. From the small batteries in your remote control to the big ones in electric cars, they all use electrochemical reactions.
- Batteries: These store chemical energy and release it as electricity when needed. They have a positive electrode (cathode) and a negative electrode (anode).
- Fuel Cells: These are like batteries, but they get their fuel from an outside source. They continuously produce electricity as long as fuel is supplied. A common example is a hydrogen fuel cell, which combines hydrogen and oxygen to make water and electricity.
Electroplating
Have you ever seen shiny chrome on a bicycle or gold-plated jewelry? That's thanks to electroplating! This process uses electricity to put a thin layer of one metal onto another. It can make things look nicer, protect them from rust, or make them stronger.
Corrosion
Sometimes, electrochemistry can be a problem. Rusting metal is an example of corrosion. This is an electrochemical process where metals react with oxygen and water. This reaction slowly damages the metal. Scientists use electrochemistry to find ways to stop or slow down corrosion.
Sensors
Many sensors use electrochemical principles. For example, the sensor that measures oxygen levels in your car's exhaust works electrochemically. Glucose meters, used by people with diabetes, also use electrochemical reactions to measure blood sugar.
History of Electrochemistry
The ideas behind electrochemistry have been around for a long time.
Early Discoveries
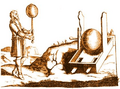
In the 17th century, scientists like Otto von Guericke experimented with static electricity. But the real breakthrough came in the late 18th century.
- Luigi Galvani: An Italian doctor, he noticed that frog legs twitched when touched by two different metals. He thought it was "animal electricity."
- Alessandro Volta: Another Italian scientist, he disagreed with Galvani. Volta believed the electricity came from the metals themselves. In 1800, he invented the voltaic pile, the first true battery. This proved that chemical reactions could make electricity.
Michael Faraday's Contributions
In the 19th century, Michael Faraday made huge discoveries. He showed the strong link between electricity and chemical reactions.
- He introduced terms like "electrode," "anode," "cathode," "ion," and "electrolyte."
- He discovered the laws of electrolysis. These laws explain how much chemical change happens when a certain amount of electricity passes through a solution. His work laid the foundation for modern electrochemistry.
Modern Electrochemistry
Later scientists built on Faraday's work.
- Svante Arrhenius: A Swedish chemist, he developed the theory of electrolytes. He explained that substances break into ions when dissolved in water.
- Walther Nernst: A German chemist, he developed the Nernst equation. This equation helps predict the voltage of an electrochemical cell.
Today, electrochemistry is a vital field. It helps us develop new batteries, create cleaner energy sources, and understand important biological processes.
Related pages
Images for kids
-
German physicist Otto von Guericke beside his electrical generator while conducting an experiment.
-
Italian physicist Alessandro Volta showing his "battery" to French emperor Napoleon Bonaparte in the early 19th century.
-
Swedish chemist Svante Arrhenius portrait circa 1880s.
-
German scientist Walther Nernst portrait in the 1910s.
See also
 In Spanish: Electroquímica para niños
In Spanish: Electroquímica para niños
 | Leon Lynch |
 | Milton P. Webster |
 | Ferdinand Smith |