Michael Faraday facts for kids
Quick facts for kids
Michael Faraday
|
|
|---|---|

Faraday c. 1826
|
|
| Born | 22 September 1791 Newington Butts, England
|
| Died | 25 August 1867 (aged 75) Hampton Court, Middlesex, England
|
| Known for | Faraday's law of induction Faraday balance Faraday cage Faraday constant Faraday cup Faraday effect Faraday's laws of electrolysis Faraday's ice pail experiment Faraday paradox Faraday paradox (electrochemistry) Faraday rotator Faraday-efficiency effect Faraday wave Faraday wheel Adsorption refrigeration Colloidal gold Homopolar motor Lines of force Magnetic separation Premelting Regelation Rubber Balloon |
| Spouse(s) |
Sarah Barnard
(m. 1821) |
| Awards | Royal Medal (1835 and 1846) Copley Medal (1832 and 1838) Rumford Medal (1846) Albert Medal (1866) |
| Scientific career | |
| Fields | Physics Chemistry |
| Institutions | Royal Institution |
| Influences | Humphry Davy William Thomas Brande |
| Influenced | James Clerk Maxwell |
| Signature | |
Michael Faraday (born September 22, 1791 – died August 25, 1867) was a brilliant English scientist. He made huge discoveries in the fields of electromagnetism (how electricity and magnetism are connected) and electrochemistry (how electricity causes chemical changes).
Faraday's most important discoveries include the basic ideas behind electromagnetic induction (making electricity with magnets), diamagnetism (how some materials are weakly pushed away by magnets), and electrolysis (using electricity to split chemical compounds). His work was super important for creating electric motors.
As a chemist, Faraday discovered a chemical called benzene. He also invented an early version of the Bunsen burner, which is still used in science labs today. He also helped popularize words like "anode", "cathode", "electrode", and "ion".
Contents
- Early Life and Learning
- Becoming a Scientist
- Later Years and Retirement
- Family Life
- Amazing Scientific Discoveries
- Work at the Royal Institution and Public Service
- Recognition and Legacy
- Remembering Michael Faraday
- Cool Facts About Michael Faraday
- Famous Quotes by Michael Faraday
- Awards Named After Faraday
- Images for kids
- See also
Early Life and Learning
Michael Faraday was born on September 22, 1791, in Newington Butts, Surrey, England. His family was not rich. His father, James, was a blacksmith. Michael was the third of four children. He had to teach himself most of what he knew.
When he was 14, Faraday started working as an apprentice for a local bookbinder and bookseller named George Riebau. This apprenticeship lasted seven years. During this time, Faraday read many books. He became very interested in science, especially in electricity.
Becoming a Scientist

In 1812, when Faraday was 20, he went to lectures by a famous chemist named Humphry Davy. In 1813, Davy had an accident that hurt his eyesight. He decided to hire Faraday as his assistant. On March 1, 1813, Faraday became a Chemical Assistant at the Royal Institution.
Later Years and Retirement

Faraday became a member of the Royal Society in 1824. This is a very important group for scientists. He was even asked twice to become its President, but he said no. In 1839, he had some health problems but got better.
In 1848, Faraday was given a house at Hampton Court to live in for free. He retired there in 1858.
Michael Faraday passed away on August 25, 1867, at 75 years old. He had refused to be buried in Westminster Abbey, a famous church where many important people are buried. However, there is a special plaque for him there, near Isaac Newton's tomb. Faraday was buried in Highgate Cemetery.
Family Life
Faraday married Sarah Barnard on June 12, 1821. They met through their church. They did not have any children.
Amazing Scientific Discoveries
Chemistry Breakthroughs

Faraday studied chlorine and found two new compounds made from chlorine and carbon. He also did early experiments on how gases spread out.
He was able to turn several gases into liquids. This showed that gases are just liquids with very low boiling points. It helped scientists understand how molecules group together. He also worked with steel and made new types of glass for optical uses.
Faraday also discovered the laws of electrolysis. These laws explain how electricity can cause chemical reactions. He also made words like anode, cathode, electrode, and ion popular.
He invented an early version of the Bunsen burner. This tool is still used in science labs around the world to provide heat. Faraday also discovered benzene, an important chemical.
In 1847, he was the first to notice something special about tiny gold particles, now called nanoparticles. He saw that their optical properties (how they interact with light) were different from larger pieces of gold. This was one of the first steps towards the field of nanoscience.
Electricity and Magnetism
Faraday is most famous for his work with electricity and magnetism.
His experiments and inventions created the basis for modern technology that uses electricity and magnetism. One of his inventions was the homopolar motor. This motor used a wire, a magnet, and a pool of mercury. When electricity flowed through the wire, it would spin around the magnet. This was the first time electrical energy was clearly shown to create continuous motion.
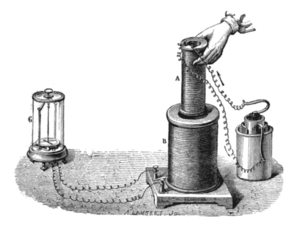
Faraday kept working in his lab, learning more about how materials behave with electricity and magnetism. In 1831, he started a series of experiments that led to his discovery of electromagnetic induction.
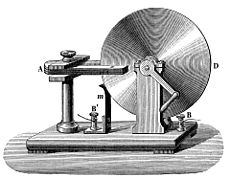
Faraday's big breakthrough happened when he wrapped two insulated wires around an iron ring. He found that when he sent electricity through one coil, a quick burst of electricity appeared in the other coil. This is called mutual induction. The iron ring he used is still at the Royal Institution. He also found that if he moved a magnet through a loop of wire, electricity would flow in the wire. The same thing happened if the wire loop moved over a still magnet.
These experiments showed that a changing magnetic field creates an electric field. This idea was later put into mathematical form by James Clerk Maxwell as Faraday's law. This law is now one of the four main Maxwell equations and is part of what we call field theory. Faraday used these ideas to build the electric dynamo, which is the ancestor of modern power generators and electric motors.

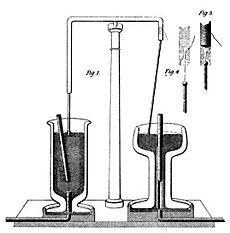
In 1832, Faraday did more experiments to understand the basic nature of electricity. He used different types of electricity, like static electricity, batteries, and "animal electricity". He showed that all these different "kinds" of electricity were actually just one single "electricity." The differences were only in how much electricity there was and how strong it was.
Diamagnetism and Light
In 1845, Faraday discovered diamagnetism. This is a phenomenon where many materials are slightly pushed away by a magnetic field.
Faraday also found that a magnetic field can change the direction of polarized light. This is called the Faraday effect.
The Faraday Cage
Michael Faraday did important experiments that helped explain how static electricity works. His ice pail experiment showed that electric charge stays on the outside of a charged object. The charge on the outside does not affect anything inside the object. This happens because the outside charges move around to protect the inside from electrical forces.
This protective effect is used in what is now called a Faraday cage. In 1836, Faraday built a large wooden frame covered with paper walls and wire mesh. He stepped inside and then electrified it. When he stepped out, he had shown that electricity was a force, not some invisible fluid, as many people believed at the time.
Work at the Royal Institution and Public Service

Faraday worked at the Royal Institution of Great Britain for a long time. He became an Assistant Superintendent in 1821. In 1825, he became the Director of the Laboratory. In 1833, he became the first Fullerian Professor of Chemistry at the Royal Institution. This was a job for life, and he didn't even have to give lectures.
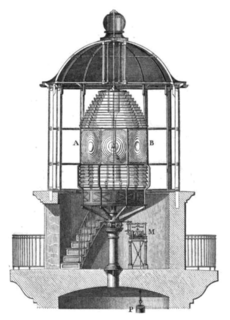
Besides his scientific research, Faraday also did many important projects for companies and the British government. This included looking into explosions in coal mines, helping with lighthouses, and protecting ships from corrosion. His workshop is still standing at Trinity Buoy Wharf in London. He did his first experiments with electric lighting for lighthouses there.
Between 1827 and 1860, Faraday gave a series of nineteen Christmas lectures for young people at the Royal Institution. These lectures continue today. The goal was to teach science to the public and inspire them.
Recognition and Legacy
- He received an honorary degree from the University of Oxford in 1832.
- He became a member of many important scientific groups around the world, including the American Academy of Arts and Sciences and the French Academy of Sciences.
Remembering Michael Faraday

There is a statue of Faraday in Savoy Place, London. The Michael Faraday Memorial is near his birthplace in Newington Butts, London. Faraday School is located at Trinity Buoy Wharf, where his workshop still stands. There's also a park called Faraday Gardens in Walworth, London.
Many buildings at universities are named after Faraday, like at London South Bank University, Loughborough University, University of Edinburgh, Brunel University, and Swansea University. There are also streets named after him in many cities around the world.

A special Royal Society of Arts blue plaque marks his former home at 48 Blandford Street in London. From 1991 to 2001, Faraday's picture was on the back of the £20 banknotes in the UK. He was shown giving a lecture at the Royal Institution.
Faraday has also been honored on postage stamps. The Faraday Institute for Science and Religion and The Faraday Institution (for battery research) are both named after him.
His life and discoveries were featured in an episode of the science show Cosmos: A Spacetime Odyssey called "The Electric Boy".
Cool Facts About Michael Faraday
- The unit of capacitance (how much electric charge a device can store) is called the farad, named after him.
- As a teenager, he was greatly inspired by a book called Conversations on Chemistry by Jane Marcet.
- He was offered a knighthood (a special honor from the King or Queen) but turned it down. He believed it went against his religious beliefs to seek worldly rewards.
- He became the first Fullerian Professor of Chemistry at the Royal Institution in 1833.
- When the government asked him to help make chemical weapons for the Crimean War, Faraday refused because of his strong ethical beliefs.
- Albert Einstein, another very famous scientist, kept a picture of Faraday on his study wall.
- In 2002, Faraday was ranked number 22 in the BBC's list of the 100 Greatest Britons.
Famous Quotes by Michael Faraday
- "A man who is certain he is right is almost sure to be wrong."
- "Nothing is ever too good to be true."
- "The important thing is to know how to take all things quietly."
- "Speculations? I have none. I am resting on certainties."
- "The book of nature which we have to read is written by the finger of God."
Awards Named After Faraday
Several awards honor Michael Faraday's amazing scientific work:
- The IET Faraday Medal
- The Royal Society of London Michael Faraday Prize
- The Institute of Physics Michael Faraday Medal and Prize
- The Royal Society of Chemistry Faraday Lectureship Prize
Images for kids
See also
 In Spanish: Michael Faraday para niños
In Spanish: Michael Faraday para niños
- Faraday (Unit of electrical charge)
- Nikola Tesla
- Tetrachloroethylene