Humphry Davy facts for kids
Quick facts for kids
Humphry Davy
|
|
|---|---|

|
|
| Born | 17 December 1778 |
| Died | 29 May 1829 (aged 50) Geneva, Switzerland
|
| Known for | Electrolysis, aluminium, sodium, potassium, calcium, strontium, magnesium, barium, boron, Davy lamp |
| Awards | Copley Medal (1805) Prix du galvanisme (1807) Rumford Medal (1816) Royal Medal (1827) |
| Scientific career | |
| Fields | Chemistry |
| Institutions | Royal Society, Royal Institution |
| Influences | Benjamin Thompson |
| Influenced | Michael Faraday, William Thomson |
| 23rd President of the Royal Society | |
| In office 1820–1827 |
|
| Preceded by | William Hyde Wollaston |
| Succeeded by | Davies Gilbert |
Sir Humphry Davy (born December 17, 1778 – died May 29, 1829) was an important British chemist and inventor. He came from Cornwall, England. Davy is famous for inventing the Davy lamp, which made coal mines much safer. He also created an early version of the arc lamp, a very bright electric light.
Davy used electricity to discover several elements for the first time. In 1807, he found potassium and sodium. The next year, he isolated calcium, strontium, barium, magnesium, and boron. He also proved that chlorine and iodine were elements, not compounds. His work on separating substances with electricity helped create a new field called electrochemistry. Davy was also the first to discover clathrate hydrates in his lab.
In 1799, he experimented with nitrous oxide, which he called "laughing gas." He noticed it made him laugh and thought it could help with pain during surgery. This was a very early idea about anaesthetics.
Davy was a highly respected scientist. He was a baronet, the President of the Royal Society, and a member of other important scientific groups.
Contents
Early Life and Learning: 1778–1798
Growing Up in Cornwall
Humphry Davy was born in Penzance, Cornwall, on December 17, 1778. He was the oldest of five children. His father, Robert Davy, was a woodcarver. Davy went to grammar school in Penzance. His family later moved to Varfell, near Ludgvan.
Davy was a very curious and independent learner. He once wrote that he was lucky to be "left much to myself as a child." He believed this helped him become who he was. His brother said Davy had a "native vigour" and a special kind of genius.
Early Science Experiments
After his father died in 1794, Davy became an apprentice to a surgeon named John Bingham Borlase. While working in the apothecary's shop, he started doing his own chemistry experiments at home. His family and friends sometimes worried about his experiments. His sister complained his chemicals were ruining her dresses!
In 1797, Davy learned French and read a famous chemistry book by Antoine Lavoisier. This book greatly influenced his future work. Davy also enjoyed writing poetry, which showed his creative side. He wrote about science, nature, and life.
Davy also learned from his friend Robert Dunkin. Dunkin showed him how rubbing two pieces of ice together could melt them. This simple experiment helped Davy understand how motion could create heat. Davy later repeated similar experiments in his lectures.
Starting His Career: 1798–1802
Discovering New Ideas
Davy met Davies Gilbert, who invited him to use his large library. This led Davy to meet Dr. Edwards, a chemistry lecturer. Edwards let Davy use his lab. Davy became interested in why copper and iron gates in the port of Hayle were decaying in seawater. This was an early look at what we now call Galvanic corrosion.
Davy also befriended Gregory Watt, the son of the famous inventor James Watt. Gregory taught Davy more about chemistry. Davy also met the Wedgwood family, who were interested in his work.
The Pneumatic Institution
In 1798, Davy joined the Pneumatic Institution in Bristol. This place was set up to study how different gases could be used in medicine. Davy was in charge of the experiments. He quickly started filling parts of the institution with electrical batteries.
While in Bristol, Davy became friends with famous writers like Samuel Taylor Coleridge and Robert Southey. They, along with James Watt, often tried nitrous oxide, or "laughing gas." Davy found that the gas made people laugh and feel happy. He also realized it might help relieve pain during surgery. However, Anesthetics were not widely used in medicine until many years after Davy's death.
Davy took risks with his experiments. Once, he inhaled carbon monoxide and nearly died. He learned from these experiences and became very careful about his experimental methods.
Joining the Royal Institution
In 1799, Benjamin Thompson, also known as Count Rumford, suggested creating the Royal Institution in London. Its goal was to spread scientific knowledge.
In February 1801, Davy was interviewed for a job there. He was hired as an assistant lecturer in chemistry and director of the lab. He also got a room to live in and a salary.
On April 25, 1801, Davy gave his first lecture on 'Galvanism' (the study of electricity). He was a fantastic speaker. His lectures were exciting, with amazing and sometimes dangerous chemical demonstrations. He also included poetry and religious ideas, which made his talks popular with everyone, including women. He believed science could help humanity.
Davy quickly became very popular. His lectures were attended by hundreds of people. By June 1802, at just 23 years old, he became a full lecturer at the Royal Institution. In November 1804, he became a Fellow of the Royal Society, a very high honor for a scientist.
Important Discoveries: 1802–1820
Early Photography Ideas
In 1802, Davy published an article about experiments he did with Thomas Wedgwood. They were trying to copy images using light and silver nitrate. They found that light could create images on paper treated with silver nitrate. Davy even thought about using a special microscope to make bigger images.
However, they couldn't find a way to make the images permanent. The pictures would fade away when exposed to light. Davy didn't spend much more time on this, but his work was an important step towards modern photography.
Discovering New Elements


Davy was a leader in using electrolysis. This is a process that uses electricity to break down chemical compounds. He used a powerful voltaic pile (an early battery) to split common substances.
In 1807, Davy discovered potassium by passing electricity through melted caustic potash. Potassium was the first metal ever isolated using electricity. In the same year, he also isolated sodium using a similar method with melted sodium hydroxide. These elements are very reactive and are called alkali metals.
In 1808, Davy continued his electrolysis experiments. He successfully isolated four more new metals: barium, calcium, strontium, and magnesium. He also isolated boron in 1809.
Davy also made a big discovery about chlorine. It was first found in 1774, but people thought it contained oxygen. In 1810, Davy proved that chlorine was actually an element itself. He named it "chlorine" from the Greek word for green-yellow, because of its color. This discovery changed how scientists understood what an acid was.
A Lab Accident and a New Assistant
Davy had a serious lab accident in 1812 while working with a dangerous chemical called nitrogen trichloride. This chemical was known to explode easily. Davy was badly injured, but his eyesight was saved. This accident led him to hire Michael Faraday as his assistant. Faraday helped him with experiments and keeping records.
Traveling in Europe

In 1812, Davy was knighted and became "Sir Humphry Davy." He also married a wealthy widow named Jane Apreece.
In 1813, Davy and his wife, along with Michael Faraday, traveled to France. Napoleon had given Davy a medal for his work in electrochemistry. Even though England and France were at war, Davy was allowed to travel because of his scientific importance.
In Paris, Davy met other famous chemists. He also studied a new substance found by Bernard Courtois, which Davy identified as the element iodine. This led to some disagreement with a French chemist about who discovered it first.
Davy's group then traveled to Italy. In Florence, he used the sun's rays to burn a diamond. This proved that diamonds are made of pure carbon. He also studied ancient paintings in Rome to learn about the chemicals used in their colors. He visited Mount Vesuvius and collected crystals.
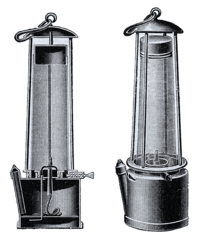
The Davy Lamp

After returning to England in 1815, Davy focused on making safer lamps for coal mines. Mine explosions caused by firedamp (methane gas) were a huge problem, killing many miners.
Davy came up with the idea of enclosing a lamp's flame with a fine wire gauze. This gauze would prevent the flame from igniting the methane in the mine air. The gas could burn safely inside the lamp without causing an explosion outside.
Other inventors, like William Reid Clanny and George Stephenson, also worked on safety lamps. Davy's use of wire gauze was a key part of many later lamp designs. Davy chose not to patent his lamp, meaning he didn't make money from it. He was given the Rumford Medal in 1816 for his invention.
Understanding Acids
In 1815, Davy also suggested a new way to define acids. He said acids were substances that contained hydrogen that could be replaced by reactive metals. When acids reacted with metals, they formed salts and hydrogen gas. He defined bases as substances that reacted with acids to form salts and water. These definitions were very useful for many years.
Later Years: 1820–1829
Protecting Ships
From 1761, the Royal Navy used copper plating on the bottom of ships to protect them from worms. But the copper would slowly wear away in salt water. Between 1823 and 1825, Davy tried to protect the copper using electrochemistry. He attached pieces of zinc or iron to the copper. These "sacrificial" metals would corrode instead of the copper.
However, there was a problem. When the copper was protected, it didn't release poisonous salts into the water. This meant that barnacles and other sea creatures could attach to the ship's hull. This slowed the ships down a lot, and captains complained. So, the Navy stopped using Davy's protectors.
President of the Royal Society
In 1820, Davy was elected President of the Royal Society. This was a very important position. The Royal Society was changing from a group of gentlemen interested in science to a more specialized scientific academy.
Davy tried to bring together different groups within the Society. He wanted to encourage new scientific fields. However, his popularity and authority decreased after the failure of his ship protection project. He also made some political mistakes.
Davy became unwell in 1826 and suffered a stroke. He traveled to Italy for his health. He was too ill to continue as president and stepped down in 1827.
Final Years and Legacy
In 1818, Davy was made a baronet, a special honor for his scientific achievements. He was the first scientist in Britain to receive such a high award.
Davy's assistant, Michael Faraday, went on to become an even more famous scientist. Some say Davy called Faraday his greatest discovery. However, Davy later accused Faraday of copying his work, which caused some tension between them.
Davy was a very enthusiastic and energetic person. He was imaginative, and some people thought he could have been a great poet if he hadn't been a chemist. His lectures were very popular because he explained things clearly and performed exciting experiments. He cared deeply about using science to help people, especially with his invention of the miners' lamp.
Davy never fully recovered from his stroke in 1826. He spent his last months writing a book called Consolations in Travel. It was a mix of poetry, science, and philosophy.
Humphry Davy died in Geneva, Switzerland, on May 29, 1829, after another stroke. He was buried in the Plainpalais Cemetery in Geneva.
Honours and Recognition
Places Named After Him
- A memorial tablet for Davy is in Westminster Abbey in London.
- A statue of Davy stands in Penzance, Cornwall, his hometown.
- Humphry Davy School in Penzance is named after him.
- A pub in Penzance is called "The Sir Humphry Davy."
- The Davy Building at the University of Plymouth is named in his honor.
- There is a road named Humphry Davy Way in Bristol.
- A giant Davy Lamp stands outside the Stadium of Light in Sunderland, honoring his invention and the local mining history.
- Davy Sound in Greenland was named after him.
Scientific and Other Honors
- In 1827, a mineral was named davyne in his honor.
- Since 1877, the Royal Society of London has given out the Davy Medal each year for important discoveries in chemistry.
- A crater on the Moon is named after him.
- Davy loved fly-fishing and is sometimes called "the father of modern fly-fishing." His book Salmonia is a very important book for fly-fishermen.
See also
 In Spanish: Humphry Davy para niños
In Spanish: Humphry Davy para niños