Boron facts for kids

boron (β-rhombohedral)
|
||||||||||||||||
| Boron | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | /ˈbɔːrɒn/ |
|||||||||||||||
| Allotropes | α-, β-rhombohedral, β-tetragonal (and more) | |||||||||||||||
| Appearance | black-brown | |||||||||||||||
| Standard atomic weight Ar, std(B) | [10.806, 10.821] conventional: 10.81 | |||||||||||||||
| Boron in the periodic table | ||||||||||||||||
|
||||||||||||||||
| Atomic number (Z) | 5 | |||||||||||||||
| Group | group 13 (boron group) | |||||||||||||||
| Period | period 2 | |||||||||||||||
| Block | p | |||||||||||||||
| Electron configuration | [He] 2s2 2p1 | |||||||||||||||
| Electrons per shell | 2, 3 | |||||||||||||||
| Physical properties | ||||||||||||||||
| Phase at STP | solid | |||||||||||||||
| Melting point | 2349 K (2076 °C, 3769 °F) | |||||||||||||||
| Boiling point | 4200 K (3927 °C, 7101 °F) | |||||||||||||||
| Density when liquid (at m.p.) | 2.08 g/cm3 | |||||||||||||||
| Heat of fusion | 50.2 kJ/mol | |||||||||||||||
| Heat of vaporization | 508 kJ/mol | |||||||||||||||
| Molar heat capacity | 11.087 J/(mol·K) | |||||||||||||||
Vapor pressure
|
||||||||||||||||
| Atomic properties | ||||||||||||||||
| Oxidation states | −5, −1, +1, +2, +3 (a mildly acidic oxide) | |||||||||||||||
| Electronegativity | Pauling scale: 2.04 | |||||||||||||||
| Ionization energies |
|
|||||||||||||||
| Atomic radius | empirical: 90 pm | |||||||||||||||
| Covalent radius | 84±3 pm | |||||||||||||||
| Van der Waals radius | 192 pm | |||||||||||||||
| Spectral lines of boron | ||||||||||||||||
| Other properties | ||||||||||||||||
| Natural occurrence | primordial | |||||||||||||||
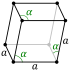
| Crystal structure | rhombohedral | |||||||||||||||
| Speed of sound thin rod | 16,200 m/s (at 20 °C) | |||||||||||||||
| Thermal expansion | β form: 5–7 µm/(m⋅K) (at 25 °C) | |||||||||||||||
| Thermal conductivity | 27.4 W/(m⋅K) | |||||||||||||||
| Electrical resistivity | ~106 Ω⋅m (at 20 °C) | |||||||||||||||
| Magnetic ordering | diamagnetic | |||||||||||||||
| Molar magnetic susceptibility | −6.7·10−6 cm3/mol | |||||||||||||||
| Mohs hardness | ~9.5 | |||||||||||||||
| CAS Number | 7440-42-8 | |||||||||||||||
| History | ||||||||||||||||
| Discovery | Joseph Louis Gay-Lussac and Louis Jacques Thénard (30 June 1808) | |||||||||||||||
| First isolation | Humphry Davy (9 July 1808) | |||||||||||||||
| Main isotopes of boron | ||||||||||||||||
|
||||||||||||||||
| 10B content may be as low as 19.1% and as high as 20.3% in natural samples. 11B is the remainder in such cases. | ||||||||||||||||
Boron is a special chemical element. Its chemical symbol is B. It has the atomic number 5. Boron is known as a metalloid. This means it has some properties like a metal and some like a non-metal.
You won't find pure boron by itself in nature. Instead, it's usually found in chemical compounds. A common place to find it is in a mineral called borax.
There are two main forms, or allotropes, of boron. One form is amorphous boron, which looks like a brown powder. The other is crystalline boron, which is black and very hard. This metallic form can conduct electricity, but not very well, especially at room temperature. Boron is the fifth element on the periodic table and is found in the Earth's crust.
Contents
What is Boron Used For?
Making Electronics Work Better
Pure boron is used in the semiconductor industry. It's added as a "dopant." A dopant is a tiny amount of a substance that changes how a semiconductor behaves with electricity. This helps make electronic devices work correctly.
Strong and Lightweight Materials
Boron compounds are very important for making strong materials that don't weigh much. This is useful in many areas, like building things that need to be both tough and light.
Safe Insecticides and Preservatives
Some boron compounds are used as non-toxic insecticides. This means they can kill insects without being harmful to people or pets. They are also used as preservatives to keep things from spoiling.
Creating New Chemicals
Boron compounds are also used in chemical synthesis. This is the process of making new chemical substances.
Boron and Living Things
Important for Plants
Plants need boron to grow and stay healthy. Even small amounts of boron are vital for their life processes.
Good for Animals
Animals, including humans, also need very small amounts of boron in their bodies. It helps keep them healthy, though scientists are still learning exactly how it works.
Discovery of Boron
Boron was first discovered by Sir Humphry Davy. He was an English chemist. He found it in the year 1808.
Boron's Properties
Boron needs a lot of heat to melt. It melts at 2075 °C (3767 °F). It boils at an even higher temperature, 4000 °C (7232 °F).
| Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hydrogen & alkali metals |
Alkaline earth metals | Triels | Tetrels | Pnictogens | Chalcogens | Halogens | Noble gases |
||||||||||||
| Period |
|||||||||||||||||||
| 2 | |||||||||||||||||||
| 3 | |||||||||||||||||||
| 4 | |||||||||||||||||||
| 5 | |||||||||||||||||||
| 6 | |||||||||||||||||||
| 7 | |||||||||||||||||||
Primordial From decay Synthetic Border shows natural occurrence of the element
- Ca: 40.078 — Abridged value (uncertainty omitted here)
- Po: [209] — mass number of the most stable isotope
Images for kids
See also
 In Spanish: Boro para niños
In Spanish: Boro para niños