Group (periodic table) facts for kids
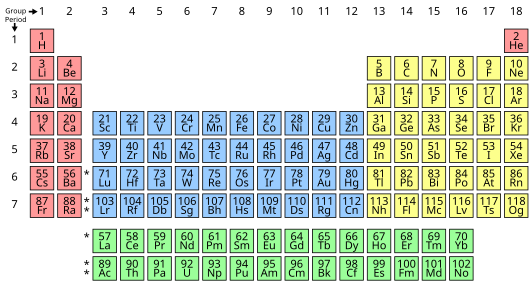
In chemistry, a group is a column of elements in the periodic table. Think of the periodic table as a big chart of all the known chemical elements. Each vertical column in this chart is called a group.
There are 18 numbered groups in the periodic table. You might notice some columns in the middle, called the f-block, are not numbered. Elements in the same group often have similar chemical properties. This is because their atoms have a similar number of electrons in their outermost shell. These outer electrons are super important for how atoms react with each other!
Scientists use a modern numbering system from 1 to 18 for these groups. This system was recommended by the International Union of Pure and Applied Chemistry (IUPAC) in 1988. Before that, there were different ways to number them, which could be a bit confusing.
Sometimes, groups are also known by the name of their first element. For example, group 16 can be called the "oxygen group." Many groups also have special names, like the "chalcogens" (also group 16) or the "halogens" (group 17).
Contents
Understanding Group Names
The most common way to name groups today is simply by numbers from 1 to 18. But many groups also have special names that are used a lot. For example, the elements in group 1 are called alkali metals, and those in group 18 are the noble gases.
Common Group Names
Here are some of the well-known groups and their special names:
- Group 1: Alkali metals (and hydrogen). These are very reactive metals.
- Group 2: Alkaline earth metals. These are also reactive metals, but a bit less than group 1.
- Group 11: Sometimes called coinage metals. This includes elements like copper, silver, and gold, which have been used to make coins.
- Group 13: The boron group.
- Group 14: The carbon group.
- Group 15: Pnictogens. This group includes nitrogen.
- Group 16: Chalcogens. This group includes oxygen.
- Group 17: Halogens. These are very reactive nonmetals, like fluorine and chlorine.
- Group 18: Noble gases. These elements are very unreactive and stable.
| IUPAC group | 1a | 2 | —groups b | 3c | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mendeleev (I–VIII) | I | II | III | IV | V | VI | VII | VIII | I | II | III | IV | V | VI | VII | d | |||
| CAS (US, A-B-A) | IA | IIA | IIIB | IVB | VB | VIB | VIIB | VIIIB | IB | IIB | IIIA | IVA | VA | VIA | VIIA | VIIIA | |||
| Old IUPAC (Europe, A-B) | IA | IIA | IIIA | IVA | VA | VIA | VIIA | VIII | IB | IIB | IIIB | IVB | VB | VIB | VIIB | 0 | |||
| Trivial namer | H and alkali metals | alkaline earth metals | triels | tetrels | pnictogens | chalcogens | halogens | noble gases | |||||||||||
| Name by elementr | lithium group | beryllium group | scandium group | titanium group | vanadium group | chromium group | manganese group | iron group | cobalt group | nickel group | copper group | zinc group | boron group | carbon group | nitrogen group | oxygen group | fluorine group | helium or neon group | |
| Period 1 | H | He | |||||||||||||||||
| Period 2 | Li | Be | B | C | N | O | F | Ne | |||||||||||
| Period 3 | Na | Mg | Al | Si | P | S | Cl | Ar | |||||||||||
| Period 4 | K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | |
| Period 5 | Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | |
| Period 6 | Cs | Ba | La–Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Period 7 | Fr | Ra | Ac–No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
b The 14 f-block groups (columns) do not have a group number.
c The correct composition of group 3 is scandium (Sc), yttrium (Y), lutetium (Lu), and lawrencium (Lr), as shown here: this is endorsed by 1988 and 2021 IUPAC reports on the question. General inorganic chemistry texts often put scandium (Sc), yttrium (Y), lanthanum (La), and actinium (Ac) in group 3, so that Ce–Lu and Th–Lr become the f-block between groups 3 and 4; this was based on incorrectly measured electron configurations from history, and Lev Landau and Evgeny Lifshitz already considered it incorrect in 1948. Arguments can still occasionally be encountered in the contemporary literature purporting to defend it, but most authors consider them logically inconsistent. Some sources follow a compromise that puts La–Lu and Ac–Lr as the f-block rows (despite that giving 15 f-block elements in each row, which contradicts quantum mechanics), leaving the heavier members of group 3 ambiguous. See also Group 3 element#Composition.
d Group 18, the noble gases, were not discovered at the time of Mendeleev's original table. Later (1902), Mendeleev accepted the evidence for their existence, and they could be placed in a new "group 0", consistently and without breaking the periodic table principle.
r Group name as recommended by IUPAC.
Old Ways to Number Groups
Before the modern 1-18 system, there were two other main ways to number groups. These systems used Roman numerals (like I, II, III) and letters (A or B).
One system was used more in Europe (called "old IUPAC"), and the other was popular in America (called "CAS"). Both systems tried to show how many outer electrons an element had. For example, potassium (K) has one outer electron, so it was in group IA in both old systems. Calcium (Ca) has two outer electrons, so it was in group IIA.
The confusing part was how they used the letters A and B.
- In the old IUPAC system, 'A' was for elements on the left and 'B' for elements on the right of the periodic table.
- In the CAS system, 'A' was for the main elements, and 'B' was for the transition metals (the ones in the middle).
Because these systems used the same names to mean different things, it could get confusing! That's why the new 1-18 system was created. It's much simpler and clearer.
Other Element Groups
While "groups" usually mean the vertical columns in the periodic table, sometimes scientists talk about other "groups" of elements that are not columns. These are just collections of elements that share similar properties.
For example:
- The noble metals are a set of metals that don't react easily, like gold and platinum.
- The refractory metals are metals that are very resistant to heat and wear.
These special names help scientists talk about elements with similar behaviors, even if they aren't in the same column.
 | Jewel Prestage |
 | Ella Baker |
 | Fannie Lou Hamer |

