Actinide facts for kids
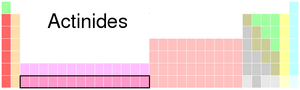
The actinide series is a special group of 15 chemical elements found on the periodic table. They are located between actinium (which has an atomic number of 89) and lawrencium (with an atomic number of 103). This whole group is named after actinium. What makes them unique is that all actinides are radioactive. This means they naturally give off energy as they change into other elements. The most common natural actinide is uranium, and thorium is the second most common.
Contents
Meet the Actinides
The table below shows all the elements that belong to the actinide series. Each element has its own unique atomic number, a chemical symbol, and a name. Some of these elements can be found naturally on Earth, while others are so rare or unstable that scientists have to create them in special laboratories.
| Atomic Number | Name | Symbol | Picture |
|---|---|---|---|
| 89 | Actinium | Ac | − |
| 90 | Thorium | Th | |
| 91 | Protactinium | Pa | − |
| 92 | Uranium | U | |
| 93 | Neptunium | Np | |
| 94 | Plutonium | Pu |  |
| 95 | Americium | Am | |
| 96 | Curium | Cm | − |
| 97 | Berkelium | Bk | |
| 98 | Californium | Cf | |
| 99 | Einsteinium | Es |  |
| 100 | Fermium | Fm | − |
| 101 | Mendelevium | Md | − |
| 102 | Nobelium | No | − |
| 103 | Lawrencium | Lr | − |
| Periodic table | |||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H | He | ||||||||||||||||||||||||||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||||||||||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||||||||||||
| Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | ||||||||||
| Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Uut | Fl | Uup | Lv | Uus | Uuo | ||||||||||
|
|||||||||||||||||||||||||||||||||||||||||
Why are Actinides Important?
Actinides are very interesting elements because of their unique properties, especially their radioactivity. This property makes them useful in many areas, from generating electricity to helping us explore space.
Energy and Power
One of the most well-known uses of actinides is in nuclear power plants. Elements like uranium and plutonium can be used as fuel. When their atoms are split in a controlled way, they release a huge amount of energy. This energy can then be used to heat water, create steam, and spin turbines to generate electricity for homes and cities.
Space Exploration
Some actinides, like plutonium-238, are used to power spacecraft that travel far into our solar system. These elements release heat as they decay, and this heat can be turned into electricity by special devices called radioisotope thermoelectric generators (RTGs). This allows probes like Cassini and Galileo to operate for many years in places where sunlight is too weak for solar panels.
Everyday Uses
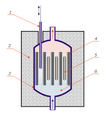
You might even find actinides in some everyday items! For example, tiny amounts of americium-241 are used in many smoke detectors. This element helps the detector sense smoke particles in the air, giving you an early warning in case of a fire.
Scientific Discoveries
Scientists continue to study actinides to learn more about how elements behave and to create new ones. Many of the heavier actinides, like Californium and Einsteinium, were first made in laboratories. These discoveries help us understand the fundamental building blocks of the universe.
Images for kids
-
Enrico Fermi suggested the existence of transuranium elements in 1934.
-
Glenn T. Seaborg and his group at the University of California at Berkeley synthesized Pu, Am, Cm, Bk, Cf, Es, Fm, Md, No and element 106, which was later named seaborgium in his honor while he was still living. They also synthesized more than a hundred actinide isotopes.
-
A pellet of 238PuO2 to be used in a radioisotope thermoelectric generator for either the Cassini or Galileo mission. The pellet produces 62 watts of heat and glows because of the heat generated by the radioactive decay (primarily α). Photo is taken after insulating the pellet under a graphite blanket for minutes and removing the blanket.
-
Interior of a smoke detector containing americium-241.
-
Self-illumination of a nuclear reactor by Cherenkov radiation.
See also
 In Spanish: Actínido para niños
In Spanish: Actínido para niños
 | Stephanie Wilson |
 | Charles Bolden |
 | Ronald McNair |
 | Frederick D. Gregory |