Graphite facts for kids
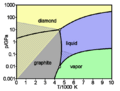
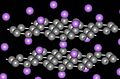
Graphite is a special form of carbon, just like diamond. Even though they are both made of carbon, their atoms are arranged differently. This difference makes them have very different properties. Graphite is made of layers of carbon atoms that can slide over each other easily. This is why it feels very soft. It looks like a dull gray rock. Because it has free-moving electrons between its layers, graphite is very good at carrying electricity.
Graphite can form when coal is put under a lot of heat and pressure deep inside the Earth. With even more heat and pressure, graphite can even turn into diamond! This is how people make synthetic, or man-made, diamonds.
You can find natural graphite in places like Sri Lanka, Canada, and the United States. People sometimes call it Lead Black because it looks a bit like the metal lead. The name "graphite" was given by Abraham Gottlob Werner in 1789. It comes from a Greek word meaning "to write."
One common use for graphite is in the "lead" of a pencil. Pencil lead is actually a mix of graphite and clay. Graphite is also used as a lubricant to help machines run smoothly.
Contents
How We Use Natural Graphite
Natural graphite is used in many different ways. It's important for things like batteries, making steel, and even pencils. Graphene, which is a super strong material, is found naturally in graphite. But it's still hard to separate it from graphite.
Graphite in Batteries
Over the last 30 years, more and more graphite has been used in batteries. Both natural and man-made graphite are used to build the inside part (called the anode) of most batteries. For example, a lithium-ion battery uses about twice as much graphite as it does lithium.
The demand for batteries has grown a lot because of portable electronics. Things like laptops, mobile phones, and tablets all need batteries. Now, electric vehicles are also increasing the need for graphite. A single electric car battery, like in a Nissan Leaf, can have almost 40 kilograms of graphite!
Graphite for Making Steel
Natural graphite is often added to hot, melted steel. This helps to increase the carbon content of the steel to the right level. Adding carbon makes the steel stronger. Graphite can also be used to lubricate the tools that shape hot steel.
Graphite in Brake Linings
Graphite is used in brake linings for larger vehicles, not usually cars. It became important when people needed to find a replacement for asbestos in brakes. Graphite helps the brakes work well.
Graphite for Molds and Lubricants
Graphite is used to make a special paint for molds in foundries. A foundry is a place where metal objects are made by pouring hot metal into molds. Painting the inside of a mold with graphite helps the finished metal object come out easily after it cools. Graphite lubricants are special. They work well in very hot or very cold places. They can be used for things like mining machines or even to lubricate locks.
Graphite in Pencils
Graphite got its name because it can leave marks on paper. The word "graphite" comes from the Ancient Greek word graphein, which means "to write or draw."
For a long time, pencils used only natural graphite. But today, most pencil "leads" are a mix of powdered graphite and clay. This mix was invented by Nicolas-Jacques Conté in 1795. It's important to remember that pencil lead has nothing to do with the metal lead. They just look similar.
Even today, pencils are a small but important use for natural graphite. About 7% of all graphite produced in 2011 was used to make pencils. The graphite used for pencils usually comes from China.
Other Uses of Graphite
Natural graphite is also used in other things. It can be found in zinc-carbon batteries and in the brushes of electric motors. Artists also use graphite because different types can create different shades and tones in drawings. Old railroads sometimes mixed powdered graphite with oil. This made a heat-resistant coating for parts of steam locomotives.
Related pages
Images for kids
-
Graphite plates and sheets, 10–15 cm high; mineral specimen from Kimmirut, Baffin Island
-
Large graphite specimen. Naturalis Biodiversity Center, Leiden, Netherlands.
-
Scanning tunneling microscope image of graphite surface
-
Alpha graphite's unit cell
See also
 In Spanish: Grafito para niños
In Spanish: Grafito para niños
 | Roy Wilkins |
 | John Lewis |
 | Linda Carol Brown |