Alkali metal facts for kids
Alkali metals are a special group of chemical elements found in the first column of the periodic table. They are known for being very reactive. This means they easily combine with other elements. Because they react so quickly, you never find them alone in nature. Instead, they are always part of compounds, like table salt.
| Atomic No. | Name | Symbol | Picture |
|---|---|---|---|
| 3 | Lithium | Li | |
| 11 | Sodium | Na | |
| 19 | Potassium | K | |
| 37 | Rubidium | Rb | |
| 55 | Caesium | Cs | |
| 87 | Francium | Fr | − |
Contents
Alkali Metals: The Reactive Elements
Alkali metals are a family of six chemical elements. They are found in Group 1 of the periodic table. These elements are lithium (Li), sodium (Na), potassium (K), rubidium (Rb), caesium (Cs), and francium (Fr).
What Makes Them Special?
Alkali metals are soft and shiny. They have a silvery-white color when they are pure. However, they quickly turn black when exposed to air. This happens because they react with oxygen in the air.
Their Unique Electron
Each alkali metal atom has only one valence electron. This is an electron in its outermost shell. This single electron makes them very eager to react. They easily give up this electron to other atoms. When they lose this electron, they get a positive charge of +1. This makes them very good at forming chemical bonds.
Why Are They So Reactive?
Alkali metals are among the most reactive elements. They react strongly with water. Because of this, they must be stored in oil. This keeps them from touching water or air.
Reactivity Trends
Lithium is the least reactive of the alkali metals. As you go down the group in the periodic table, the elements become more reactive. For a long time, scientists thought francium was the most reactive. However, it is very rare. More recent studies suggest that caesium is actually more reactive than francium.
Important Alkali Metals
Sodium is the most common and important alkali metal. You probably know it best from sodium chloride (NaCl). This is the common 'table salt' we use every day. Sodium also forms sodium hydroxide (NaOH). This is a very strong chemical called 'caustic soda'. It is used in many industries.
| Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hydrogen & alkali metals |
Alkaline earth metals | Triels | Tetrels | Pnictogens | Chalcogens | Halogens | Noble gases |
||||||||||||
| Period |
|||||||||||||||||||
| 2 | |||||||||||||||||||
| 3 | |||||||||||||||||||
| 4 | |||||||||||||||||||
| 5 | |||||||||||||||||||
| 6 | |||||||||||||||||||
| 7 | |||||||||||||||||||
Primordial From decay Synthetic Border shows natural occurrence of the element
- Ca: 40.078 — Abridged value (uncertainty omitted here)
- Po: [209] — mass number of the most stable isotope
Images for kids
-
Johann Wolfgang Döbereiner was among the first to notice similarities between what are now known as the alkali metals.
-
Dmitri Mendeleev's periodic system proposed in 1871 showing hydrogen and the alkali metals as part of his group I, along with copper, silver, and gold
-
Estimated abundances of the chemical elements in the Solar system. Hydrogen and helium are most common, from the Big Bang. The next three elements (lithium, beryllium, and boron) are rare because they are poorly synthesised in the Big Bang and also in stars. The two general trends in the remaining stellar-produced elements are: (1) an alternation of abundance in elements as they have even or odd atomic numbers, and (2) a general decrease in abundance, as elements become heavier. Iron is especially common because it represents the minimum energy nuclide that can be made by fusion of helium in supernovae.
-
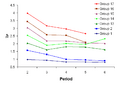
The variation of Pauling electronegativity (y-axis) as one descends the main groups of the periodic table from the second to the sixth period
-
A reaction of 3 pounds (≈ 1.4 kg) of sodium with water
-
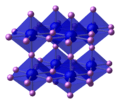
Unit cell ball-and-stick model of lithium nitride. On the basis of size a tetrahedral structure would be expected, but that would be geometrically impossible: thus lithium nitride takes on this unique crystal structure.
-
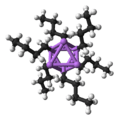
Solid phenyllithium forms monoclinic crystals can be described as consisting of dimeric Li2(C6H5)2 subunits. The lithium atoms and the ipso carbons of the phenyl rings form a planar four-membered ring. The plane of the phenyl groups are perpendicular to the plane of this Li2C2 ring. Additional strong intermolecular bonding occurs between these phenyllithium dimers and the π electrons of the phenyl groups in the adjacent dimers, resulting in an infinite polymeric ladder structure.
-
Similarly to the alkali metals, ammonia reacts with hydrochloric acid to form the salt ammonium chloride.
-
Very pure thallium pieces in a glass ampoule, stored under argon gas
-
A wheel type radiotherapy device which has a long collimator to focus the radiation into a narrow beam. The caesium-137 chloride radioactive source is the blue square, and gamma rays are represented by the beam emerging from the aperture. This was the radiation source involved in the Goiânia accident, containing about 93 grams of caesium-137 chloride.
See also
 In Spanish: Alcalino para niños
In Spanish: Alcalino para niños
 | William M. Jackson |
 | Juan E. Gilbert |
 | Neil deGrasse Tyson |