Mendelevium facts for kids
Mendelevium (pronounced /ˌmɛndəˈlɛviəm/) is a special kind of metal that scientists have made. It is not found naturally on Earth. Its symbol is Md, and its atomic number is 101. This means it has 101 protons in its atoms. Mendelevium is also radioactive, which means it gives off energy. It belongs to a group of elements called actinides. Scientists created mendelevium by smashing einsteinium with tiny particles called alpha particles. It was named after Dmitri Mendeleev, a famous scientist who created the Periodic Table of elements.
| Periodic table | |||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H | He | ||||||||||||||||||||||||||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||||||||||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||||||||||||
| Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | ||||||||||
| Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Uut | Fl | Uup | Lv | Uus | Uuo | ||||||||||
|
|||||||||||||||||||||||||||||||||||||||||
Contents
Discovering Mendelevium
Mendelevium was first made in 1955. A team of scientists at the University of California, Berkeley discovered it. This team included Albert Ghiorso, Glenn T. Seaborg, Bernard Harvey, Gregory R. Choppin, and Stanley G. Thompson. They used a special machine called a cyclotron.
How Scientists Made It
To create mendelevium, the scientists used a very small amount of einsteinium. They bombarded this einsteinium with alpha particles. Alpha particles are like the nucleus of a helium atom. When the einsteinium atoms absorbed these alpha particles, they changed into new, heavier atoms: mendelevium.
A Tiny Amount of a New Element
The discovery was amazing because they only made about 17 atoms of mendelevium. This was a very small amount! They had to work quickly because mendelevium is very unstable. It quickly breaks down into other elements. This means it has a very short half-life. The most stable form of mendelevium, called an isotope, has a half-life of only 51 days. Other forms last only a few minutes or even seconds.
Why is it Called Mendelevium?
The element was named after Dmitri Mendeleev. He was a Russian chemist who lived from 1834 to 1907. Mendeleev is famous for creating the Periodic Table of Elements. This table organizes all the known elements based on their properties. Naming an element after him was a way to honor his huge contribution to chemistry.
What is the Periodic Table?
The Periodic Table is like a map of all the elements. It helps scientists understand how elements are related. It also helps them predict what new elements might be like. Mendeleev's table was a breakthrough. It showed that elements have repeating patterns in their behavior.
Properties of Mendelevium
Mendelevium is a synthetic element. This means it does not exist in nature. It can only be made in a laboratory. It is also a radioactive element. This means its atoms are unstable and release energy as they break down.
What are Actinides?
Mendelevium is part of a group called the actinides. These are a series of 15 metallic chemical elements. They are found at the bottom of the Periodic Table. All actinides are radioactive. They include elements like uranium and plutonium.
Uses of Mendelevium
Because mendelevium is so rare and unstable, it has no practical uses outside of scientific research. Scientists study it to learn more about the properties of very heavy elements. This helps them understand how atoms work and how new elements can be created.
Studying Superheavy Elements
Research into elements like mendelevium helps scientists explore the limits of the Periodic Table. It also helps them understand the forces that hold the nucleus of an atom together. This kind of research can lead to new discoveries in nuclear physics.
Images for kids
-
The 60-inch cyclotron at the Lawrence Radiation Laboratory, University of California, Berkeley, in August 1939
-
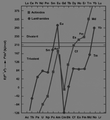
Energy required to promote an f electron to the d subshell for the f-block lanthanides and actinides. Above around 210 kJ/mol, this energy is too high to be provided for by the greater crystal energy of the trivalent state and thus einsteinium, fermium, mendelevium form divalent metals like the lanthanides europium and ytterbium. (Nobelium is also expected to form a divalent metal, but this has not yet been confirmed.)
See also
 In Spanish: Mendelevio para niños
In Spanish: Mendelevio para niños
 | Jessica Watkins |
 | Robert Henry Lawrence Jr. |
 | Mae Jemison |
 | Sian Proctor |
 | Guion Bluford |