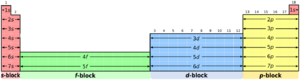
Block (periodic table) facts for kids
Imagine the periodic table as a big map of all known elements. A block on this map is a group of elements that are similar because of where their outermost electrons are found. These special electron "homes" are called atomic orbitals.
The idea of blocks was first used by a scientist named Charles Janet. Each block gets its name from the type of orbital its electrons occupy: the s-block, p-block, d-block, f-block, and even a predicted g-block.
The letters s, p, d, and f come from old terms used to describe light from atoms: "sharp," "principal," "diffuse," and "fundamental." These terms help scientists understand the energy levels of electrons. If new blocks are discovered, they would follow the alphabet, like g, h, and so on.
Contents
Understanding Periodic Table Blocks
What Are Blocks?
The division into blocks is justified by their distinctive nature: s is characterized, except in H and He, by highly electropositive metals; p by a range of very distinctive metals and non-metals, many of them essential to life; d by metals with multiple oxidation states; f by metals so similar that their separation is problematic. Useful statements about the elements can be made on the basis of the block they belong to and their position in it, for example highest oxidation state, density, melting point ... Electronegativity is rather systematically distributed across and between blocks.
In Foundations of Chemistry, 2017
The way elements are grouped into blocks is based on their electron setup. This also generally matches how they behave chemically. For example, the s-block and p-block elements are often called the "main-group" elements. The d-block elements are known as transition metals. The f-block elements are the "inner transition metals." This group includes almost all the lanthanides (like lanthanum and uranium) and actinides.
Some elements, like zinc, cadmium, and mercury (from group 12), are sometimes seen as main-group elements. This is because their chemical behavior is more like p-block elements than other d-block elements. Similarly, group 3 elements can sometimes seem like s-block elements. But remember, they are still officially d-block elements.
You might notice that the columns (groups) in the f-block don't have numbers. This is a unique feature of this block.
Helium is a special case. Its only electrons are in the s-orbital, making it an s-block element. However, it acts more like the noble gases in the p-block (group 18) because its electron shell is full.
The s-Block Elements
Na, K, Mg and Ca are essential in biological systems. Some ... other s-block elements are used in medicine (e.g. Li and Ba) and/or occur as minor but useful contaminants in Ca bio-minerals e.g. Sr…These metals display only one stable oxidation state [+1 or +2]. This enables [their] ... ions to move around the cell without…danger of being oxidized or reduced.
The role of calcium and comparable cations in animal behaviour, RSC, Cambridge, p. 1
The s-block is on the left side of the periodic table. The "s" stands for "sharp." This block includes elements from the first two columns, plus hydrogen and helium. It contains the alkali metals (group 1) and alkaline earth metals (group 2).
These elements usually have one or two electrons in their outermost s-orbital. Even though helium is an s-element, it's usually placed with the noble gases in group 18 because it has a full outer shell.
Most s-block metals are soft and have low melting and boiling points. Many of them can create colorful flames when heated. Except for helium, all s-block elements are very reactive. They often form strong bonds with nonmetals, especially with elements like the halogens.
The p-Block Elements
The p-block is on the right side of the periodic table, covering groups 13 to 18. The "p" stands for "principal." These elements have their outermost electrons in p-orbitals. Each row of the table, except the very first, has space for six p-elements.
This block is special because it contains all three types of elements: metals, nonmetals, and metalloids. You might know some of these groups by their special names: group 17 are the halogens, and group 18 (excluding helium) are the noble gases.
A p-orbital can hold up to six electrons. This is why the p-block has six columns. Elements in the first p-block column (group 13) have one p-orbital electron. Elements in the second column (group 14) have two, and so on, up to six electrons in group 18.
Many p-block elements follow the octet rule, meaning they try to have eight electrons in their outer shell. However, elements in later rows can sometimes hold more than eight electrons. These elements can also have different oxidation states, which means they can form different types of chemical bonds. Generally, elements in a p-block group become less reactive as you go down the column.
The d-Block Elements
The ... elements show a horizontal similarity in their physical and chemical properties as well as the usual vertical relationship. This horizontal similarity is so marked that the chemistry of the first ... series ... is often discussed separately from that of the second and third series, which are more similar to one another than to the first series.
Chemistry: Facts, patterns, and principles, Addison-Wesley, London, pp. 487−489
The d-block is in the middle of the periodic table, covering groups 3 to 12. The "d" stands for "diffuse." This block starts in the fourth row (period) of the table. Each row from the fourth onwards has space for ten d-block elements.
Most of these elements are called transition metals. They are like a bridge between the very reactive metals of groups 1 and 2, and the less reactive metals of groups 13 to 16. Elements in group 3 or group 12 are d-block metals, but sometimes they are not called transition metals. This is because they don't always show the same chemical behaviors, like having many different oxidation states.
All d-block elements are metals. Most have one or more electrons in their d-orbitals that are active in chemical reactions. Because these d-orbital electrons have similar energy levels, the number of electrons involved in bonding can change. This allows d-block elements to have two or more oxidation states. The most common ones are +2 and +3.
The d-orbitals have interesting shapes, some like four-leaf clovers. They can hold up to ten electrons.
The f-Block Elements
Because of their complex electronic structure, the significant electron correlation effects, and the large relativistic contributions, the f-block elements are probably the most challenging group of elements for electronic structure theory.
Computational method in lanthanide and actinide chemistry, John Wiley & Sons, Chichester, p. xvii
The f-block, where "f" stands for "fundamental," is usually shown as a separate section at the bottom of the periodic table. If you look at a wider table, it's actually in the middle-left, between groups 2 and 3. This block starts in the sixth row (period). Each row from the sixth onwards has space for fourteen f-block elements.
These elements are often called inner transition metals. They act as a transition between the s-block and d-block in the 6th and 7th rows. This is similar to how d-block metals bridge the s-block and p-block in earlier rows.
The f-block has two series of elements: the lanthanides (from lanthanum to ytterbium) in period 6, and the actinides (from actinium to nobelium) in period 7. All of them are metals.
In the lanthanides, the f-orbital electrons are less involved in chemical reactions, making these elements quite similar to each other. For the early actinides, the f-orbital electrons are more active. This means these elements can show a wider range of chemical behaviors. Later actinides, from curium onwards, behave more like the lanthanides.
The f-orbitals have complex shapes and can hold up to fourteen electrons. This is why the f-block takes up fourteen columns in a full periodic table. These elements are not given group numbers because their vertical trends are not as clear as in other blocks.
For a long time, there was some confusion about which elements truly belonged to the f-block. However, based on strong scientific evidence, the International Union of Pure and Applied Chemistry (IUPAC) confirmed that the f-block includes elements from lanthanum to ytterbium, and from actinium to nobelium.
The Predicted g-Block
A g-block is predicted to exist for very heavy elements, starting around element 121. The "g" stands for the next letter in the alphabetical order after f.
Scientists believe that g-orbitals might start to fill with electrons around element 124 to 126. These g-orbitals would likely begin to play a role in the chemistry of these elements.
If the pattern of previous blocks continued, the g-block would have eighteen elements. However, calculations suggest that the eighth period might not follow the usual patterns as closely.
See Also
 | Georgia Louise Harris Brown |
 | Julian Abele |
 | Norma Merrick Sklarek |
 | William Sidney Pittman |