Inner transition metal facts for kids
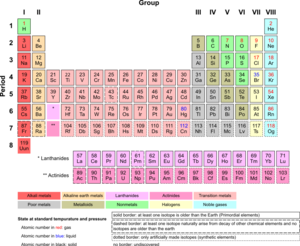
Inner transition metals are a special group of chemical elements. You can find them on the periodic table. They are usually shown in two separate rows at the very bottom. These elements are all metals.
Contents
What are Inner Transition Metals?
Inner transition metals are a unique family of elements. They are all metals. They have three outer electron shells that are not completely full. This gives them special properties. These elements can be quite soft and bendable. This means they are often malleable (can be hammered into thin sheets) and ductile (can be pulled into wires).
These elements belong to the "f-block" on the periodic table. This means their special electrons are found in an "f" orbital.
Lanthanides: The Rare Earths
The first group of inner transition metals is called the lanthanides. These elements have atomic numbers from 57 to 71. They are also sometimes called "rare earth elements." However, many of them are not actually rare. They are just hard to find in large, pure amounts.
Lanthanides are very similar to each other. They often have similar chemical behaviors. Many lanthanides, like Lutetium, are used in the lighting industry. They help make bright, energy-efficient lights. They are also used in things like lasers and magnets.
Actinides: The Radioactive Metals
The second group of inner transition metals is called the actinides. These elements have atomic numbers from 89 to 103. A very important thing about actinides is that they are all radioactive. This means they give off energy as they break down.
Most actinides do not exist naturally on Earth. Only thorium and uranium are found in nature. The other actinides are made by scientists in laboratories. Because they are radioactive, actinides are often used in nuclear power plants. They are also used in some medical treatments and scientific research. Actinides are known for being very unstable. This means they change and break down quickly.
Images for kids
 | Shirley Ann Jackson |
 | Garett Morgan |
 | J. Ernest Wilkins Jr. |
 | Elijah McCoy |