Ionization energy facts for kids
Ionization energy is a super important idea in chemistry! It's all about how much energy you need to pull an electron away from an atom. Imagine an atom holding onto its electrons; ionization energy is the "tug" needed to make one let go. This atom is usually all by itself, not connected to any other atoms.
Contents
What is Ionization Energy?
Every atom has a nucleus (its center) and tiny electrons spinning around it. These electrons are held in place by an electrical pull from the nucleus. Ionization energy is the amount of energy it takes to completely remove one of these electrons from an atom. Think of it like trying to pull a magnet off a fridge – some are easier to pull off than others!
Why is Ionization Energy Important?
Understanding ionization energy helps scientists predict how chemical elements will react with each other. Elements with low ionization energy tend to lose electrons easily, which makes them very reactive. Elements with high ionization energy hold onto their electrons tightly and are less likely to give them up.
Ionization Energy and the Periodic Table
The periodic table is a super helpful chart that organizes all the known chemical elements. Ionization energy follows some cool patterns on this table:
Across the Periodic Table
As you move from left to right across a row (called a "period") on the periodic table, the ionization energy generally increases. This means it takes more energy to remove an electron. Why? Because atoms on the right side have more protons in their nucleus, which creates a stronger pull on their electrons, holding them tighter.
Down the Periodic Table
As you move down a column (called a "group") on the periodic table, the ionization energy generally decreases. This means it takes less energy to remove an electron. Why? Because atoms further down a group have more electron "shells" or layers. This means their outermost electrons are farther away from the nucleus. The farther away an electron is, the weaker the pull from the nucleus, making it easier to remove.
Removing More Than One Electron
What happens if you want to remove a second electron, or even a third? It gets harder each time! The energy needed to remove the first electron is called the "first ionization energy." The energy to remove the second electron is the "second ionization energy," and so on. Each time you remove an electron, the remaining electrons are pulled even closer by the nucleus, making it much tougher to remove the next one.
Images for kids
-
The added electron in boron occupies a p-orbital.
-
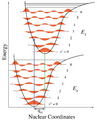
Figure 1. Franck–Condon principle energy diagram. For ionization of a diatomic molecule, the only nuclear coordinate is the bond length. The lower curve is the potential energy curve of the neutral molecule, and the upper curve is for the positive ion with a longer bond length. The blue arrow is vertical ionization, here from the ground state of the molecule to the v=2 level of the ion.
See also
 In Spanish: Energía de ionización para niños
In Spanish: Energía de ionización para niños