History of the battery facts for kids
Batteries are amazing devices that give us electricity! Before big power plants and electrical grids existed, batteries were the main way people got electricity. Over time, batteries got better and better. These improvements helped create many important things, from early scientific experiments to the first telegraphs and telephones. Today, batteries power our portable computers, mobile phones, electric cars, and countless other gadgets we use every day.
Some batteries are called "wet cells." These were open containers with liquid chemicals and metal parts. When the metal parts were used up, you had to replace them and the liquid. Wet cells weren't good for things you carried around, but they were used in early telegraph and telephone systems. Some early electric cars even used them!
Batteries can also be sorted by how long they last. "Primary" batteries make electricity right away, but once their chemicals are used up, you can't recharge them. Think of the batteries in your TV remote – you throw them away when they die. But then, the "secondary" or "rechargeable" battery was invented, like the lead–acid battery. These batteries can have their energy restored by plugging them in! Later, nickel and lithium batteries came along, making it possible to create all sorts of portable electronics, from powerful flashlights to smartphones. Very large batteries are even used to help store energy for big power grids, making sure our electricity supply is steady.
Contents
How Batteries Were Invented
Before batteries, people used Leyden jars to store electrical charge. These were like early capacitors, holding electricity physically and releasing it all at once. Scientists would connect several Leyden jars to get a stronger zap. One famous inventor, Benjamin Franklin, might have been the first to call his group of jars an "electrical battery," like how soldiers use a group of cannons together!
Volta's Amazing Pile
A scientist named Alessandro Volta was friends with Luigi Galvani, who had some interesting findings about electricity. Volta thought that electricity happened when two different metals touched a moist material. He tested this idea and published his results in 1791.
In 1800, Volta invented the first real battery! It stored and released electricity using a chemical reaction, not just by holding a charge. This invention was called the voltaic pile. It was made of copper and zinc discs stacked on top of each other, separated by cloth or cardboard soaked in salty water (which is called an electrolyte).
Unlike the Leyden jar, Volta's pile made a steady flow of electricity. It didn't lose much power when not in use. He tried different metals and found that zinc and silver worked best.

Volta first thought that the electricity came just from the metals touching. But scientists later realized that the zinc plates were getting used up faster when more electricity was drawn. This showed that the chemical reactions were actually super important for the battery to work!
Volta's first batteries had some problems. The liquid could leak out, causing short circuits because the weight of the discs squeezed the wet cloth. A Scotsman named William Cruickshank fixed this by laying the battery parts in a box instead of stacking them. This was called the trough battery. Volta also invented a version called the Crown of Cups, which was a chain of cups filled with salt water, linked by metal arcs made of two different metals.
Another problem was that Volta's batteries didn't last very long, sometimes only an hour! This was because hydrogen bubbles would form on the copper, stopping the electricity flow. Also, tiny short-circuits would happen around impurities in the zinc, making the zinc wear away. In 1835, William Sturgeon solved the zinc problem by treating it with mercury.
Even with their flaws, Volta's batteries were a huge step forward. They provided a much steadier current than Leyden jars and led to many new discoveries, like the first time water was split into hydrogen and oxygen using electricity.
First Useful Batteries
The Daniell Cell
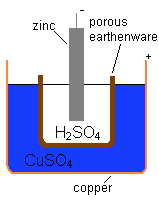
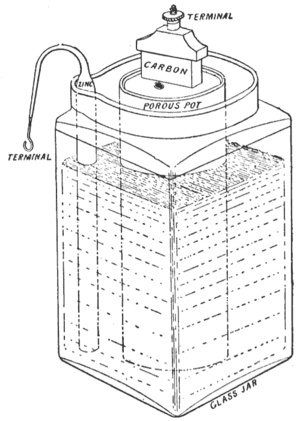
An English chemistry professor named John Frederic Daniell found a way to solve the hydrogen bubble problem in Volta's battery. In 1836, he invented the Daniell cell. This battery used a copper pot filled with a copper sulfate solution. Inside that, there was an unglazed clay pot filled with sulfuric acid and a zinc electrode. The clay pot was porous, meaning it had tiny holes that let charged particles (ions) pass through, but kept the liquids from mixing.
The Daniell cell was a big improvement! It was the first truly practical source of electricity. It gave a longer and more reliable current than Volta's battery. It was also safer and less corrosive. It produced about 1.1 volts of electricity. Soon, it became the standard battery for new telegraph networks. It was even used as the first official standard for defining the "volt," which is the unit of electrical force.
- Bird's Cell: In 1837, Golding Bird made a version of the Daniell cell using a plaster of Paris barrier.
- Porous Pot Cell: In 1838, John Dancer created another version where the zinc electrode was in a porous pot with zinc sulfate, and this pot was placed in a copper can with copper sulfate.
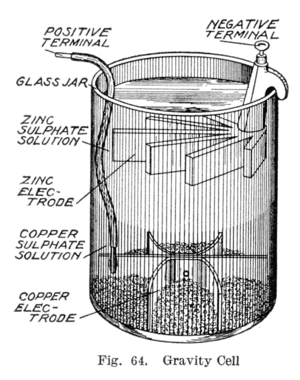
The Gravity Cell
In the 1860s, a Frenchman named Callaud invented a simpler Daniell cell called the gravity cell. This one didn't need a porous barrier! This made the battery produce a stronger current. It quickly became the favorite battery for American and British telegraph networks and was used widely until the 1950s.
The gravity cell was a glass jar with a copper part at the bottom and a zinc part hanging from the top. Copper sulfate crystals were put at the bottom, and then the jar was filled with distilled water. As electricity was used, a layer of zinc sulfate formed at the top around the zinc. This top layer stayed separate from the blue copper sulfate layer because it was lighter and because of the battery's electrical forces.
The clear zinc sulfate layer let technicians easily see how much life the battery had left. However, this battery could only be used in one place, because if you moved it, the liquids would mix or spill. Also, it needed to be used constantly, or the liquids would mix over time, so it wasn't good for on-and-off use.
Poggendorff Cell
In 1842, Johann Christian Poggendorff created a cell that mixed the chemicals together, getting rid of the porous pot. This cell used dilute sulfuric acid and chromic acid. It had two carbon plates as the positive side and a zinc plate in between them as the negative side. Because the acid mixture would react with the zinc, there was a way to lift the zinc plate out of the acids when the battery wasn't being used.
This cell provided 1.9 volts and was popular with experimenters because of its high voltage and steady current. But it was fragile and needed the zinc plate to be lifted, so it eventually became less popular. It was also known as the 'chromic acid cell' or 'bichromate cell'.
Grove Cell
William Robert Grove invented the Grove cell in 1839. It had a zinc part in sulfuric acid and a platinum part in nitric acid, separated by porous clay. The Grove cell gave a high current and almost twice the voltage of the Daniell cell, making it popular for telegraph networks for a while. However, it released poisonous nitric oxide fumes when used, and its voltage dropped quickly as it ran out of power. Also, platinum is very expensive.
Rechargeable Batteries and Dry Cells
The First Rechargeable Battery: Lead-Acid
Before this, all batteries would stop working permanently once their chemicals were used up. But in 1859, Gaston Planté invented the lead–acid battery, which was the first battery that could be recharged! You could send electricity back through it to reverse the chemical reactions.
A lead-acid cell has a lead negative part and a lead dioxide positive part, both in sulfuric acid. Both parts react with the acid to make lead sulfate, but the lead part releases electrons while the lead dioxide part uses them, creating electricity. These reactions can be reversed by recharging the battery.
Planté's first model was two lead sheets separated by rubber strips and rolled into a spiral. His batteries were first used to power lights on trains when they stopped at a station. In 1881, Camille Alphonse Faure made an improved version that was easier to mass-produce.
Lead-acid batteries are quite heavy and big for the amount of energy they hold. However, they can produce very large bursts of electricity because they have low internal resistance. This means one battery can power many things at once.
The lead-acid battery is still used today in cars and other places where weight isn't a big problem. The basic idea hasn't changed since 1859. In the 1970s, "sealed" versions became common, which meant the battery could be used in different positions without leaking.
Today, batteries that can't be recharged are called "primary" cells. Batteries that can be recharged are called "secondary" cells. The lead-acid battery was the first "secondary" cell.
The Leclanché Cell
In 1866, Georges Leclanché invented a battery with a zinc negative part and a manganese dioxide positive part (mixed with a little carbon for better conductivity), all dipped in a jar of ammonium chloride solution. It produced 1.4 volts. This battery quickly became popular for telegraphs, signals, and electric bells.
The dry cell version of this battery was used to power early telephones, often in a wooden box next to the phone. The Leclanché cell couldn't provide a steady current for very long. During long phone calls, the battery would weaken, making the conversation hard to hear. This happened because chemical reactions inside the battery would increase its internal resistance, lowering the voltage.
The First Dry Cell: Zinc-Carbon
Many inventors tried to make battery liquids solid so they would be easier to use. In 1886, Carl Gassner got a German patent for a version of the Leclanché cell that became known as the dry cell because it didn't have a free liquid. Instead, the ammonium chloride was mixed with plaster of Paris to make a paste, with a little zinc chloride added to make it last longer. The manganese dioxide positive part was dipped in this paste, and both were sealed in a zinc shell, which also acted as the negative part.
Unlike earlier wet cells, Gassner's dry cell was more solid, didn't need maintenance, didn't spill, and could be used in any direction. It produced 1.5 volts. The first mass-produced model was the Columbia dry cell, sold by the National Carbon Company in 1896. They improved Gassner's design by using coiled cardboard instead of plaster of Paris, which left more space for the positive part and made the battery easier to put together. This was the first convenient battery for everyone and made portable electrical devices possible, leading directly to the invention of the flashlight.
The zinc–carbon battery is still made today! Other inventors like Wilhelm Hellesen and Sakizō Yai also developed their own dry cell designs around the same time.
NiCd: The First Alkaline Battery
In 1899, a Swedish scientist named Waldemar Jungner invented the nickel–cadmium battery, which was rechargeable. It used nickel and cadmium electrodes in a potassium hydroxide solution. This was the first battery to use an alkaline electrolyte (a different type of chemical solution). It was sold in Sweden in 1910 and came to the United States in 1946. The first models were strong and held more energy than lead-acid batteries, but they were much more expensive.
20th Century: New Batteries Everywhere!
| Size | Year introduced |
|---|---|
| D | 1898 |
| AA | 1907 |
| AAA | 1911 |
| 9V | 1956 |
Nickel-Iron Batteries
Waldemar Jungner also patented a nickel–iron battery in 1899, but he thought it wasn't as good as his nickel-cadmium one. It produced more hydrogen gas when charging, so it couldn't be sealed, and charging was less efficient.
Thomas Edison saw a chance to make money in the battery market. In the 1890s, he worked on an alkaline battery, hoping that if he made a light and strong battery, electric cars would become popular, and his company would be the main battery supplier. After many experiments, he patented an alkaline nickel–iron battery in 1901. However, his first model leaked and didn't last much longer than lead-acid batteries. Even though Edison made a better model seven years later, by then the affordable Model T Ford had made gasoline cars the standard. Still, Edison's battery was very successful in other areas, like electric trains, backup power for railroad signals, and lights in mines.
Common Alkaline Batteries
Until the late 1950s, the zinc–carbon battery was very popular, but its short life limited sales. A Canadian engineer named Lewis Urry, working for Union Carbide, was asked to find a way to make zinc-carbon batteries last longer. Instead, he decided that alkaline batteries had more promise. Until then, longer-lasting alkaline batteries were too expensive. Urry's battery used a manganese dioxide positive part and a powdered zinc negative part with an alkaline electrolyte. Using powdered zinc gave the negative part a larger surface area. These batteries were first sold in 1959 and are still very common today!
Nickel-Hydrogen and Nickel Metal-Hydride Batteries
The nickel–hydrogen battery was first used in commercial communication satellites to store energy.
The first nickel–metal hydride batteries (NiMH) for everyday use appeared in 1989. These were a newer version of the nickel-hydrogen battery. NiMH batteries usually last longer than NiCd batteries (and they keep getting better as new materials are found). Also, since cadmium is toxic, NiMH batteries are better for the environment.
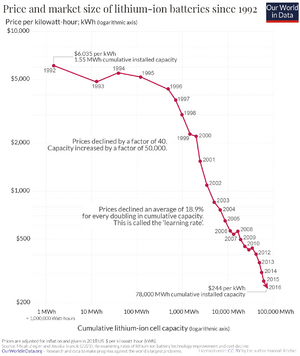
Lithium-Ion Batteries
Lithium is a very light metal with great electrical potential, meaning it can store a lot of energy for its weight. Its small ions also move quickly, making it an ideal battery material. Scientists started experimenting with lithium batteries in 1912, but commercial lithium batteries didn't appear until the 1970s as the lithium-ion battery. Small 3-volt lithium batteries are still widely used, especially in cameras and tiny devices.
Three important things happened with lithium batteries in the 1980s:
- In 1980, John B. Goodenough discovered the LiCoO2 (Lithium cobalt oxide) positive electrode, and Rachid Yazami discovered the graphite negative electrode.
- In 1981, Tokio Yamabe and Shizukuni Yata discovered a new carbon material that worked very well for the negative electrode.
- This led a team led by Akira Yoshino in Japan to build the first lithium-ion battery prototype in 1985. This was a rechargeable and more stable version of the lithium battery. Sony started selling lithium-ion batteries in 1991.
In 2019, John Goodenough, Stanley Whittingham, and Akira Yoshino won the Nobel Prize in Chemistry for their work on lithium-ion batteries!
In 1997, the lithium polymer battery was released. These batteries hold their electrolyte in a solid material instead of a liquid. This allows the battery to be put into a flexible wrapping instead of a hard metal case, meaning they can be shaped to fit specific devices. This is why lithium polymer batteries are great for mobile phones, personal digital assistants, and radio-controlled aircraft, as they allow for more flexible and compact designs. They usually hold a bit less energy than regular lithium-ion batteries.
Because lithium can be expensive and there are concerns about mining it, people are now looking into sodium-ion battery development, with some electric vehicles starting to use them in 2023.
Images for kids
See also
 In Spanish: Historia de la pila para niños
In Spanish: Historia de la pila para niños
- Baghdad Battery, an old artifact that seems similar to a modern battery
- Memory effect
- Comparison of commercial battery types
- History of electrochemistry
- List of battery sizes
- List of battery types
- Search for the Super Battery, a 2017 film about batteries
- Burgess Battery Company
 | William Lucy |
 | Charles Hayes |
 | Cleveland Robinson |