Zinc–carbon battery facts for kids
A zinc–carbon battery is a type of dry cell battery. It's a "primary battery," meaning you use it once and then throw it away. These batteries make electricity from a chemical reaction. This reaction happens between zinc (a metal) and manganese dioxide (a chemical powder). They are mixed with a special liquid called an ammonium chloride electrolyte.
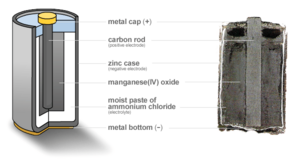
A zinc-carbon battery usually makes about 1.5 volts of electricity. The zinc part is the negative side (called the anode), and it's often the battery's outer case. Inside, there's a carbon rod surrounded by manganese dioxide, which is the positive side (called the cathode). The name "zinc-carbon" can be a bit confusing because the carbon doesn't actually create the electricity; the manganese dioxide does.
Some general-purpose zinc-carbon batteries use a wet paste made of ammonium chloride as the electrolyte. They also have some zinc chloride solution on a paper separator. This separator acts like a "salt bridge" to help the electricity flow. "Heavy-duty" types of these batteries mostly use a paste made of zinc chloride.
Zinc-carbon batteries were the first dry batteries sold. They were a big deal because they made it possible to have flashlights and other portable devices. They were cheaper and held more energy than older batteries. Today, they are still good for things that don't need a lot of power, like remote controls, clocks, or small transistor radios. However, newer and more efficient alkaline batteries have mostly replaced them.
Contents
How Zinc-Carbon Batteries Started
The idea for these batteries came from an older "wet" battery called the Leclanché cell, which was improved in 1876. Then, in 1886, a scientist named Carl Gassner created a "dry" version. He used a zinc metal case for the negative part and a paste (first plaster, then graphite powder) for the inside.
In 1898, Conrad Hubert used these new dry batteries to power the very first flashlight. He then teamed up with the battery maker to form the Eveready Battery Company. By 1900, Gassner showed off his dry cells for portable lights at a big fair in Paris.
Over the years, zinc-carbon batteries got much better. By the end of the 1900s, they could hold four times more power than they did in 1910! Improvements included using purer manganese dioxide and adding graphite powder to help electricity flow better. They also got better seals to prevent leaks and used purer zinc.
Older batteries used to have a tiny bit of mercury on the zinc. This helped protect the zinc from reacting too quickly. But mercury is bad for the environment, so modern batteries don't use it. Now, manufacturers use very pure zinc to keep the battery working well and prevent it from losing power when not in use.
As of 2011, zinc-carbon batteries still made up about 20% of all portable batteries in the United Kingdom and 18% in the European Union.
What's Inside a Zinc-Carbon Battery?
The outside of a zinc-carbon dry cell is a zinc can. This zinc can is the negative part of the battery. Inside the can, there's a paper layer soaked in ammonium chloride paste. This paste is the electrolyte. The paper separator keeps the zinc can from touching the positive part, which would cause a short circuit.
The positive part is a mix of powdered carbon (usually graphite) and manganese (IV) oxide. This mix is packed around a carbon rod. Carbon is used for the rod because most other metals would rust quickly when touching the salty electrolyte.
Older, cheaper batteries used a separator made of starch or flour. Modern batteries use a thinner paper separator coated with starch. This allows more manganese dioxide to be used, which means more power.
Originally, batteries were sealed with a sticky material called asphalt to stop the electrolyte from drying out. Now, a plastic seal is used. This seal helps prevent leaks and holds in any gas that might build up inside. The carbon rod is a bit porous, which helps absorb some of the gases produced. The amount of carbon and manganese dioxide in the paste changes how the battery works. More carbon means less internal resistance, while more manganese dioxide means more storage capacity.
Some batteries are made of flat cells stacked together. These can create much higher voltages, sometimes up to 450 volts. The whole stack is covered in wax to stop the electrolyte from drying out. When you use a device, electricity flows from the negative zinc part to the positive carbon part through the device's wires.
What Are They Used For?
Zinc-carbon batteries are cheaper than some other types. Because of this, they are often used in things that don't need a lot of power. This includes things like remote controls for TVs, clocks, and smoke detectors. They were also commonly used in old hand-cranked telephone magneto phones to power the microphone and speaker.
How They Make Electricity
In a zinc-carbon battery, the outer zinc container is the negative terminal. The zinc reacts with the chemicals inside to create electricity. This process is called oxidation. The manganese dioxide and other chemicals react at the carbon rod, which is the positive terminal. This process is called reduction. These reactions create an electromotive force (e.m.f.) of about 1.5 V. Over time, as the chemicals are used up, the battery's power can drop, especially when it's powering something.
Heavy-Duty Zinc-Chloride Cells
The zinc-chloride cell is an improved version of the regular zinc-carbon battery. It's often called "heavy-duty," "extra-heavy-duty," or "super-heavy-duty." These batteries use purer chemicals. This means they last longer and give a more steady voltage. They can last about twice as long as regular zinc-carbon cells, or even four times longer in devices that use a lot of power.
However, even these "heavy-duty" batteries don't last as long as Alkaline batteries. Alkaline batteries can last up to eight times longer than zinc-carbon batteries, especially in devices that use a lot of power or run continuously.
Storing Your Batteries
Manufacturers suggest storing zinc-carbon batteries at room temperature. If they get too hot, they won't last as long. You can freeze zinc-carbon batteries without damaging them. But it's best to let them warm up to room temperature before using them. Also, make sure no water condenses on the battery when it warms up. Over the years, the storage life of these batteries has gotten much better.
How Long Do They Last?
Zinc-carbon batteries don't last very long, even when you're not using them. This is because the ammonium chloride inside slowly reacts with the zinc casing. As the battery is used, or even just sits there, the zinc case gets thinner. When it gets too thin, the chemicals inside can start to leak out. This can make the battery sticky.
This picture shows new batteries (a) and used batteries (b) and (c). The battery at (c) had a plastic film to help keep the chemicals inside.
What Happens When You Throw Them Away?
How you throw away batteries depends on where you live. For example, in California in the U.S., all batteries are considered hazardous waste when thrown out. You can't just put them in the regular trash. In Europe, there are special rules for battery disposal. Zinc-carbon batteries should not be thrown away with regular household trash. In the EU, most stores that sell batteries must also take old batteries back for recycling.

See also
 In Spanish: Pila de zinc-carbono para niños
In Spanish: Pila de zinc-carbono para niños
- Comparison of battery types
- List of battery sizes
- List of battery types
- Photoflash battery
 | Ernest Everett Just |
 | Mary Jackson |
 | Emmett Chappelle |
 | Marie Maynard Daly |