Dry cell facts for kids
A dry cell is a special kind of electric battery. It's super common in portable electronic devices, like remote controls or flashlights.
The dry cell was invented in 1886 by a German scientist named Carl Gassner. Before that, batteries were "wet cells" and often spilled liquid. A Japanese inventor, Yai Sakizo, made a modern version in 1887.
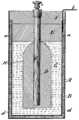
Unlike older "wet cells," a dry cell uses a paste-like substance called an electrolyte. This paste has just enough moisture for electricity to flow. Because there's no free liquid, a dry cell won't spill. This makes it perfect for things you carry around. Old wet cells were often fragile glass containers that could easily break and spill.
A common type of dry cell is the zinc-carbon cell. It usually provides about 1.5 volts of power. Another popular one is the alkaline cell, which also gives 1.5 volts. Both use a mix of zinc and manganese dioxide to create electricity.
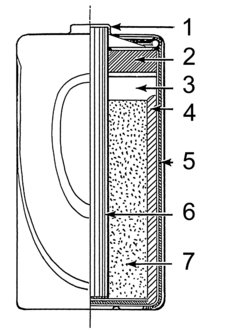
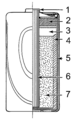
Inside a standard dry cell, you'll find a zinc outer case, which acts as the anode (the negative part). In the middle, there's a carbon rod, which is the cathode (the positive part). The space between them is filled with a paste. This paste is usually made of ammonium chloride and manganese dioxide. The manganese dioxide helps the battery work better by stopping unwanted reactions. Some "heavy duty" dry cells use zinc chloride instead of ammonium chloride.
Types of Dry Cells
There are two main types of dry cells:
Primary Cells
Primary cells are like one-time-use batteries. Once the chemicals inside have been used up, you throw them away. You can't recharge them.
Secondary Cells
Secondary cells are different because you can recharge them! This means you can use them many times before they need to be replaced.
Related pages
Images for kids
See also
 In Spanish: Pila seca para niños
In Spanish: Pila seca para niños
 | Delilah Pierce |
 | Gordon Parks |
 | Augusta Savage |
 | Charles Ethan Porter |