Leclanche battery facts for kids
A Leclanché battery is a type of battery that you cannot recharge. It was invented by a French engineer named Georges Leclanché in 1866. This battery used to be very common, but today, the alkaline battery has mostly replaced it. Leclanché batteries give out less power than alkaline ones, but they are cheaper to make. They are also lighter than alkaline batteries and can often be found in less expensive stores.
These batteries are sometimes sold with labels like "Extra Heavy Duty" or "Super Heavy Duty." This means some parts inside have been changed to make them last longer. A Leclanché battery works by a chemical process where zinc reacts with manganese dioxide. It uses a special liquid or paste called an electrolyte, which is usually made of zinc chloride or ammonium chloride.
Contents
What is a Leclanché Battery?
A Leclanché battery is known as a "primary cell." This means that once its chemical reactions are finished, it stops working and cannot be recharged. You simply throw it away and use a new one. This is different from rechargeable batteries, like those in your phone or laptop.
How Does a Leclanché Battery Work?
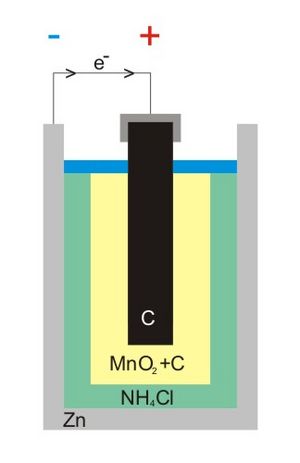
Inside a Leclanché battery, there are a few main parts:
- A carbon rod in the center, which acts as the positive side (cathode).
- A paste of manganese dioxide and carbon powder around the carbon rod.
- A zinc container that acts as the negative side (anode).
- An electrolyte, which is a liquid or moist paste that helps electricity flow. It's usually made of ammonium chloride or zinc chloride.
When the battery is used, the zinc metal slowly breaks down in a process called oxidation. This creates electrons that flow out of the battery and power your device. These electrons then return to the battery and react with the manganese dioxide. This chemical reaction creates electricity.
The Invention of the Leclanché Battery
The Leclanché battery was invented by Georges Leclanché in 1866. He was a French electrical engineer. His invention was very important because it was one of the first practical batteries that could be used outside of a laboratory. It quickly became popular for many uses, especially in early telegraph systems and doorbells.
Leclanché vs. Alkaline Batteries
Today, you might find Leclanché batteries, but alkaline batteries are much more common. Here's how they compare:
- Power: Alkaline batteries can provide more power and last longer for devices that need a lot of energy, like digital cameras or toys with motors. Leclanché batteries are better for low-power devices.
- Cost: Leclanché batteries are generally cheaper to make and buy than alkaline batteries.
- Weight: Leclanché batteries are often lighter than alkaline batteries of the same size.
- Uses: Because they are cheaper, Leclanché batteries are often used in simple, low-drain devices like remote controls, clocks, or cheap flashlights. Alkaline batteries are used in more demanding electronics.
Other Pages
Images for kids
See also
 In Spanish: Pila Leclanché para niños
In Spanish: Pila Leclanché para niños
 | Emma Amos |
 | Edward Mitchell Bannister |
 | Larry D. Alexander |
 | Ernie Barnes |