History of electrochemistry facts for kids
Electrochemistry is a super cool part of chemistry that studies how electricity and chemical reactions are connected. It's all about how electricity can make chemicals change, and how chemical changes can create electricity!
This field has come a long way. It started with early ideas about magnets and electricity in the 1500s. Now, it helps us understand things like how electricity moves, electric charge, and even uses math to solve problems.
Today, electrochemistry is super important for many modern technologies. It helps us make better batteries and fuel cells that power our devices and cars. It also helps stop metals from rusting (which is called corrosion). Plus, it's used to clean dirty water and make chemicals purer using methods like electrolysis.
Contents
Early Discoveries About Electricity and Magnets
The 1500s and 1600s were exciting times for science. People started to really understand electricity and magnetism. This knowledge eventually led to the invention of electric power and the industrial revolution in the late 1800s.
In the 1550s, an English scientist named William Gilbert spent 17 years doing experiments. He focused mostly on magnetism and a bit on electricity. Because of his amazing work with magnets, he's known as "The Father of Magnetism." His book, De Magnete, became the go-to guide in Europe for understanding electricity and magnetism. He clearly showed that magnetism was different from the "amber effect" (which we now call static electricity).
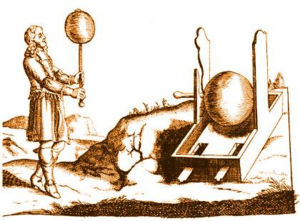
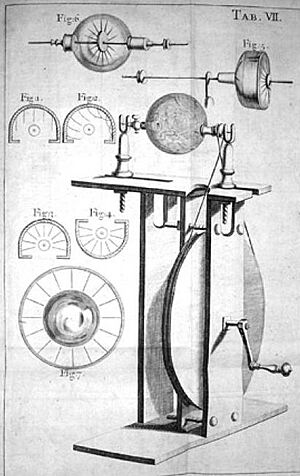
In 1663, a German physicist named Otto von Guericke built the very first machine that could create static electricity. It worked by rubbing things together. His machine had a big ball made of sulfur inside a glass globe. This globe was on a spinning rod. When he turned a crank, the ball spun, and if he rubbed a pad against it, it made a static electric spark! He could even take the globe off and use it to do other electricity experiments. Von Guericke used his generator to show that objects with the same electric charge push each other away.
The 1700s and the Start of Electrochemistry
In 1709, Francis Hauksbee in London made an interesting discovery. He put a little bit of mercury inside Von Guericke's generator and removed the air. He found that the globe would glow whenever it built up a charge and his hand touched it. He had accidentally created the first gas-discharge lamp!
Between 1729 and 1736, two English scientists, Stephen Gray and Jean Desaguliers, did some cool experiments. They showed that you could electrify an object, like a cork, from far away (up to 900 feet or 275 meters!). They did this by connecting it to a charged glass tube using metal wires or hemp string. They also found that some materials, like silk, wouldn't carry the electric effect.
By the mid-1700s, a French chemist named Charles François de Cisternay Du Fay made an important discovery about static electricity. He found there were two types, and that charges that were alike pushed each other away, while unlike charges pulled each other closer. Du Fay believed electricity was made of two "fluids": vitreous (positive) and resinous (negative). This was called the "two-fluid theory." Later, Benjamin Franklin came up with a "one-fluid theory."
In 1748, Nollet invented one of the first tools to measure electric charge, called an electrometer. It showed electric charge using how objects attracted or repelled each other. He also gave the name "Leyden jar" to the first device that could store electricity.
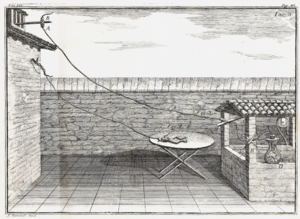
By the 1740s, William Watson tried to figure out how fast electricity traveled. People thought it was faster than sound, but no one had measured it. Watson set up a long wire, over 12,000 feet (3.7 km) long, in a field. Even at that length, electricity seemed to travel instantly!
By the 1750s, electricity became very popular, and people wanted better ways to make it. The generator made by Jesse Ramsden was one of the first good ones. The electricity from these generators was even used to treat health problems like paralysis and muscle spasms.
Charles-Augustin de Coulomb developed an important law in 1781 about how electric charges attract and repel each other. He invented a special device to measure these electric forces. He also found that the force between charges gets weaker the further apart they are (this is called the inverse square law). He wrote many important papers on electricity and magnetism. The unit of electric charge, the coulomb, is named after him.
In 1789, Franz Aepinus created a device like a "condenser" (what we now call a capacitor). It was the first capacitor after the Leyden jar. It helped show how electricity moves and how it can be induced.
Even with all these discoveries, the real start of electrochemistry came in the late 1700s. An Italian physician named Luigi Galvani found a link between muscle movements and electricity. In 1791, he wrote about his experiments where he proposed a "nerve-electrical substance" in living things.
Galvani believed that animal tissue had a special, unknown force he called "animal electricity." He thought this force made muscles move when they were touched by two different metal probes. He thought this was a new type of electricity, different from lightning or static electricity. He felt the brain made this "electric fluid," and nerves carried it to the muscles.
Galvani's ideas were mostly accepted, but Alessandro Volta, a physics professor, wasn't so sure. He thought the frog legs in Galvani's experiments were just acting like a meter for electricity. Volta believed that the contact between two different metals was the real source of the electricity. He called this "metallic electricity." Volta's name was later used for the unit of electrical potential, the volt.
Electrochemistry Becomes a Branch of Chemistry
In 1800, English chemists William Nicholson and Johann Wilhelm Ritter successfully split water into hydrogen and oxygen using electricity. This process is called electrolysis. Soon after, Ritter discovered electroplating, which is using electricity to coat one metal with another. He also noticed that the amount of metal deposited and oxygen produced depended on how far apart the electrodes (the parts where electricity enters or leaves) were.
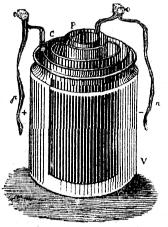
In 1802, William Cruickshank designed the first electric battery that could be made in large numbers. He arranged square copper plates and zinc plates together in a wooden box filled with salty water or weak acid. This design was better than Volta's because it didn't dry out and provided more energy.
Scientists like William Hyde Wollaston and Smithson Tennant worked on better ways to purify platinum using electrochemistry. Tennant discovered the elements iridium and osmium. Wollaston, in turn, discovered the metals palladium in 1803 and rhodium in 1804.
Wollaston also improved the galvanic battery (named after Galvani) in the 1810s. His battery used a U-shaped copper plate and a zinc plate in a pot, making it easier to use.
In 1809, Samuel Thomas von Soemmering created the first telegraph. He used 26 wires, one for each letter, connected to a container of acid. When electricity flowed through a wire, it caused the acid to bubble at a specific spot, letting him send messages one letter at a time.
Humphry Davy's work with electrolysis led him to believe that electricity in simple cells came from chemical reactions between the metals and the liquid they were in. He thought that using electric currents was the best way to break down all substances into their basic elements. His work directly led to him isolating elements like sodium, potassium, and other alkaline earth metals in 1808.
Hans Christian Ørsted made a huge discovery in 1820: electric currents create magnetic effects! This was a big step forward. André-Marie Ampère quickly studied Ørsted's experiment and put it into mathematical rules (which became Ampère's law). Ørsted also found that not only does a magnetic needle move near an electric current, but the electric wire itself moves in a magnetic field. This laid the groundwork for building electric motors.
In the 1820s, Robert Hare developed the Deflagrator, a powerful battery used for quick and strong burning. He even used it in 1831 for blasting underwater with electricity.
In 1821, Thomas Johann Seebeck discovered that if you connect two different metals and heat one of the connection points, it creates an electric current. He didn't realize it was an electric current at first, calling it "thermomagnetic currents." This Seebeck effect is now the basis of the thermocouple, which is used to measure temperature very accurately.
In 1827, German scientist Georg Ohm published his famous book where he explained his complete theory of electricity, including what we now call Ohm's law.
In 1829, Antoine-César Becquerel developed a "constant current" cell, which was a step towards the well-known Daniell cell. He also found that shining light on an electrode in a liquid could create an electric current. This was an early step towards solar cell technology!
Michael Faraday started experiments in 1832 to prove that all types of electricity worked the same way. He made two big discoveries. First, electricity didn't just act from a distance; it had to pass through a liquid to cause chemical changes. Second, the amount of chemical change was directly related to the amount of electricity that passed through the solution.
These findings led Faraday to a new theory of electrochemistry. He said that electricity created tension in the molecules of a solution. When this tension was strong enough, the molecules would break apart and move in opposite directions. This showed that the amount of electricity was linked to how chemicals reacted. These experiments led to Faraday's two laws of electrochemistry:
- The amount of a substance that forms on an electrode is directly related to how much electricity goes through the cell.
- The amounts of different elements that form from a certain amount of electricity are related to their chemical equivalent weights (how much of each element reacts).
William Sturgeon built an electric motor in 1832 and invented the commutator, a part that helps electric motors keep spinning in one direction. He also improved batteries.
Hippolyte Pixii, a French instrument maker, built the first dynamo (a machine that makes electricity) in 1832. This was the first practical machine to generate electric current using Faraday's ideas.
John Daniell started experiments in 1835 to make better batteries. In 1836, he invented a battery that solved the problem of polarization (where the battery's power drops over time). His battery was the first to produce a steady and reliable electric current for a long time.
William Grove created the first fuel cell in 1839. He knew that electricity could split water into hydrogen and oxygen. So, he tried to reverse the process: combining hydrogen and oxygen to make electricity and water. The term fuel cell was later coined in 1889. Grove's battery was very powerful and was used in early American telegraphs.
As telegraphs became more complex, a steady voltage was very important. Grove's battery had limitations, so the Daniell battery became more popular. In 1841, Robert Bunsen replaced the expensive platinum electrode in Grove's battery with a cheaper carbon electrode. This led to the widespread use of the "Bunsen battery" for things like arc-lighting and electroplating.
Wilhelm Weber developed the electrodynamometer in 1846, a device that measures electric current. In 1852, he defined the unit of electrical resistance, which was named the ohm after Georg Ohm. Weber's name is also used for the unit of magnetic flux, the weber.
German physicist Johann Hittorf figured out that electric current is caused by ion movement. In 1853, he noticed that some ions (charged atoms or molecules) moved faster than others. This led to the idea of "transport number," which describes how much electric current specific ions carry.
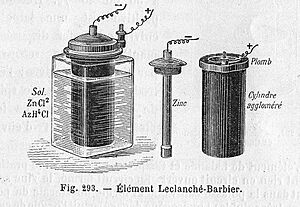
In 1866, Georges Leclanché patented a new battery system that quickly became very popular. His battery had a positive electrode (the cathode) made of manganese dioxide and carbon, and a negative electrode (anode) made of a zinc rod. These were placed in a solution of ammonium chloride, which acted as the electrolyte. Leclanché's "wet" cell was the ancestor of the first widely used battery, the zinc-carbon cell.
Late 1800s and Modern Electrochemistry
In 1869, Zénobe Gramme invented his first direct current dynamo, a machine that made smooth, steady electricity.
Svante August Arrhenius published his important work in 1884 about how electrolytes conduct electricity. He concluded that when electrolytes dissolve in water, they break apart into positive and negative ions. He believed these ions carried the electric current and were responsible for chemical activity.
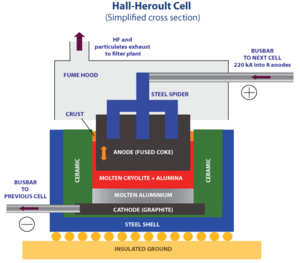
The race to produce aluminium cheaply was won in 1886 by Paul Héroult and Charles M. Hall. The problem was that using electrolysis on aluminum salt in water just made aluminum hydroxide. Hall and Héroult solved this by dissolving aluminum oxide in a special melted salt called cryolite. This process is still used today to make aluminum!
Wilhelm Ostwald, who won a Nobel Prize in 1909, started his work in 1875. He studied how water reacts and how chemicals combine, focusing on electrochemistry. In 1894, he gave the first modern definition of a catalyst (something that speeds up a chemical reaction without being used up). He's known for his important studies on how organic acids conduct electricity and break apart into ions.
Hermann Nernst developed a theory for the electromotive force (the "push" that makes electricity flow) of batteries in 1888. He also found that liquids with high dielectric constants (a measure of how well a material stores electrical energy) help substances break into ions. In 1889, he explained how batteries work by imagining an "electrolytic pressure" that pushes ions from the electrodes into the solution. He also showed how the electricity produced could be used to calculate the energy change in the chemical reaction. He created the Nernst Equation, which describes a battery cell's voltage based on its properties.
In 1898, Fritz Haber published a textbook on electrochemistry. He wanted to connect chemical research to industrial processes. He also showed that specific products could be made by controlling the voltage at the cathode during electrolytic oxidation and reduction.
In 1909, Robert Andrews Millikan started experiments to find the electric charge of a single electron. He first used charged water droplets, but they evaporated too quickly. In 1910, he got more accurate results with his famous oil-drop experiment, using oil instead of water.
Jaroslav Heyrovský, a Nobel laureate, found a much easier way to analyze chemicals. Before him, people had to weigh mercury drops. In 1921, Heyrovský had the idea to measure the current flowing through the cell instead.
On February 10, 1922, the "polarograph" was invented! Heyrovský recorded how current changed with voltage for a solution. He correctly understood that an increase in current meant that Na+ ions were being deposited. Soon after, he and his Japanese colleague, Masuzo Shikata, built the first machine to automatically record these curves, which became famous as the polarograph.
In 1923, Johannes Nicolaus Brønsted and Thomas Martin Lowry published similar theories about how acids and bases behave, using electrochemical ideas.
The International Society of Electrochemistry (ISE) was founded in 1949. A few years earlier, in 1937, Arne Tiselius developed the first advanced electrophoretic machine. He won a Nobel Prize in 1948 for his work on protein electrophoresis. He created a method called zone electrophoresis to separate proteins in a solution. Electrophoresis became widely used in the 1940s and 1950s to separate many different molecules.
During the 1960s and 1970s, quantum electrochemistry was developed by Revaz Dogonadze and his students.
See also
 In Spanish: Historia de la electroquímica para niños
In Spanish: Historia de la electroquímica para niños
- Electrochemistry
- History of the battery
- Karpen Pile