Hydrogen halide facts for kids
Hydrogen halides are special chemical compounds. They are made up of two main parts: a hydrogen atom and a halide atom. Think of them like a team of two!
The "halide" part comes from a group of elements called halogens. These include fluorine, chlorine, bromine, and iodine. Another halogen, astatine, doesn't form a stable hydrogen halide, so we usually don't include it in this group.
Hydrogen halides are diatomic molecules, meaning they have two atoms joined together. When they are in a gas form, they don't easily break apart into separate charged particles (ions). This is why chemists make a difference between, for example, hydrogen chloride gas and hydrochloric acid. Hydrogen chloride is a gas at room temperature. When this gas mixes with water, it forms hydrochloric acid. Once the acid is formed, it's not easy to turn it back into the original gas molecule.
Contents
What are the main hydrogen halides?
There are four main hydrogen halides that are important to know about:
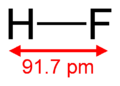
Hydrogen Fluoride (HF)
This compound has one hydrogen atom and one fluorine atom.
- Formula: HF
- Bond length: 91.7 pm (picometers)
- Dipole: 1.86 D (Debye)
- Notes: It is a very strong acid and can be quite dangerous.
Hydrogen Chloride (HCl)
This is made of one hydrogen atom and one chlorine atom.
- Formula: HCl
- Bond length: 127.4 pm
- Dipole: 1.11 D
- Notes: This is a strong acid and is the most common hydrogen halide you might hear about.
Hydrogen Bromide (HBr)
This compound has one hydrogen atom and one bromine atom.
- Formula: HBr
- Bond length: 141.4 pm
- Dipole: 0.788 D
- Notes: It is also a strong acid.
Hydrogen Iodide (HI)
This is made of one hydrogen atom and one iodine atom.
- Formula: HI
- Bond length: 160.9 pm
- Dipole: 0.382 D
- Notes: This is a strong acid and is also known as a reducing agent. This means it can help other chemicals gain electrons in a reaction.
Images for kids
See also
 In Spanish: Haluro de hidrógeno para niños
In Spanish: Haluro de hidrógeno para niños