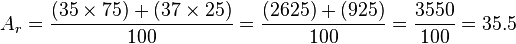Atomic mass facts for kids
An atomic mass (symbol: ma) is the mass of a single atom of a chemical element. It includes the masses of the three tiny parts that make up an atom: protons, neutrons, and electrons.
Atoms are incredibly small, so their masses are also very tiny. Because of this, we don't usually measure atomic mass in grams. Instead, we use a special unit called the unified atomic mass unit (unit symbol: u). One unified atomic mass unit is defined as exactly 1/12 of the mass of a single carbon-12 atom. This means a carbon-12 atom has a mass of 12 u.
Because electrons are so light, we can mostly think of the mass of an atom coming from its protons and neutrons. In a carbon-12 atom, there are 6 protons and 6 neutrons. Since their masses are almost the same, we can say that both protons and neutrons each have a mass of roughly 1 u.
Contents
What is Atomic Mass?
Atomic mass tells us how heavy one specific atom is. It's the total weight of all the protons, neutrons, and electrons in that atom. Even though electrons are part of the atom, their mass is so small that protons and neutrons contribute almost all of an atom's mass.
Mass Number: A Quick Estimate
The mass number (symbol: A) of an atom is simply the total count of its protons and neutrons in the nucleus. It's always a whole number and doesn't have any units. Since protons and neutrons each weigh about 1 u, the mass number gives us a good, quick estimate of an atom's atomic mass in unified atomic mass units. Usually, the atomic mass of an atom is very close to its mass number, often within 0.1 u.
What are Isotopes?
The number of protons in an atom is what makes it a specific element. For example, all carbon atoms have 6 protons. However, atoms of the same element can have different numbers of neutrons. An atom of an element with a certain number of neutrons is called an isotope.
For example, the element chlorine has two common isotopes: chlorine-35 and chlorine-37. Both have 17 protons. But chlorine-35 has 18 neutrons, while chlorine-37 has 20 neutrons (two more!). Each isotope has its own unique atomic mass, which we call its isotopic mass. So, chlorine-35 has an isotopic mass of about 35 u, and chlorine-37 has an isotopic mass of about 37 u.
Relative Atomic Mass: The Average Weight
When you look at a sample of an element, it usually contains a mix of its different isotopes. For example, natural chlorine is about 75% chlorine-35 and 25% chlorine-37.
The relative atomic mass (symbol: Ar) is the average mass of all the atoms of an element in a typical sample. It takes into account how common each isotope is. Like relative isotopic mass, it's a ratio and has no units.
To find the relative atomic mass, we calculate a weighted average of the isotopic masses. For our chlorine example:
So, the relative atomic mass of chlorine is 35.5. This average is what you usually see on the periodic table.
Related pages
See Also
 In Spanish: Masa atómica para niños
In Spanish: Masa atómica para niños