Oxygen difluoride facts for kids
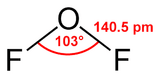
Oxygen difluoride is a special chemical compound. Its chemical formula is OF2. It looks a bit like water (H2O) because it has a V-shape. But OF2 is very different from water in how it acts. It is a strong oxidizer, which means it can make other chemicals lose electrons.
How Oxygen Difluoride is Made
Scientists first made oxygen difluoride in 1929. They created it using a process called electrolysis. This involved melting potassium fluoride and hydrofluoric acid together. There was also a little bit of water in the mixture.
Today, chemists make OF2 in a different way. They mix fluorine gas with a weak solution of sodium hydroxide in water. When these chemicals react, oxygen difluoride is formed. Another chemical, sodium fluoride, is also made during this reaction.
Here is the chemical reaction:
- 2 F2 + 2 NaOH → OF2 + 2 NaF + H2O
What Oxygen Difluoride Does
Oxygen difluoride is a very powerful oxidizer. This is because the oxygen atom in OF2 has an unusual oxidation number of +2. This is quite rare for oxygen.
If you heat OF2 above 200 °C, it breaks down. It turns into oxygen gas and fluorine gas.
OF2 can react with many different materials.
- It reacts with metals to form oxides and fluorides.
- It also reacts with nonmetals. For example, when it meets phosphorus, it forms PF5 and POF3.
- If it reacts with sulfur, it creates SO2 and SF4.
- Even a noble gas like xenon can react with OF2. This reaction produces XeF4 and other xenon oxyfluorides.
Oxygen difluoride reacts very slowly with water. When it does, it forms hydrofluoric acid and oxygen gas.
- OF2 (aq) + H2O (aq) → 2 HF (aq) + O2 (g)
It can also oxidize sulfur dioxide (SO2) to make sulfur trioxide (SO3).
- OF2 + SO2 → SO3 + F2
However, if UV light is present, the reaction changes. Then, it forms different chemicals like sulfuryl fluoride (SO2F2) and pyrosulfuryl fluoride (S2O5F2).
- OF2 + 2 SO2 → S2O5F2
Safety Information
Oxygen difluoride is a dangerous chemical. It is very highly oxidizing, which means it can react strongly and sometimes violently with other substances. Because of this, it must be handled with extreme care by trained professionals.
Quick facts for kids Oxygen difluoride |
|
|---|---|
 |
|
 |
|
| Other names | difluorine monoxide fluorine monoxide oxygen fluoride hypofluorous anhydride |
| Identifiers | |
| CAS number | |
| PubChem | |
| RTECS number | RS2100000 |
| SMILES | FOF |
|
InChI
InChI=1/F2O/c1-3-2
|
|
| Properties | |
| Molecular formula | |
| Molar mass | 0 g mol-1 |
| Appearance | colorless gas, pale yellow liquid when condensed |
| Density | 1.9 g/cm3 |
| Melting point | |
| Boiling point | |
| Solubility in other solvents | 68 mL gaseous OF2 in 1 L (0 °C) |
| Thermochemistry | |
| Std enthalpy of formation ΔfH |
24.5 kJ mol−1 |
| Related compounds | |
| Related compounds | HFO O2F2 NHF2 NF3 SCl2 |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
See also
 In Spanish: Difluoruro de oxígeno para niños
In Spanish: Difluoruro de oxígeno para niños
 | Jessica Watkins |
 | Robert Henry Lawrence Jr. |
 | Mae Jemison |
 | Sian Proctor |
 | Guion Bluford |

