Phosphorus pentafluoride facts for kids
Quick facts for kids Phosphorus pentafluoride |
|
|---|---|
 |
|
 |
|
| IUPAC name | Phosphorus pentafluoride |
| Other names | Phosphorus(V) fluoride Pentafluoridophosphorus Pentafluorophosphorane |
| Identifiers | |
| CAS number | |
| PubChem | |
| RTECS number | TH4070000 |
| Properties | |
| Molecular formula | |
| Molar mass | 0 g mol-1 |
| Appearance | colourless gas |
| Density | 5.527 g/cm3 |
| Melting point | |
| Boiling point | |
| hydrolysis | |
| Structure | |
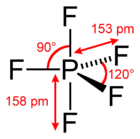
| Molecular shape | trigonal bipyramidal |
| Dipole moment | 0 D |
| Hazards | |
| EU Index | Not listed |
| Flash point | Non-flammable |
| Related compounds | |
| Other anions | Phosphorus pentachloride Phosphorus pentabromide Phosphorus pentaiodide |
| Other cations | Arsenic pentafluoride Antimony pentafluoride Bismuth pentafluoride |
| Related compounds | Phosphorus trifluoride |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Phosphorus pentafluoride, also known as PF5, is a special chemical compound. It is made from the elements phosphorus and fluorine. At normal room temperature and pressure, it is a gas that you cannot see because it has no color.
Contents
What is Phosphorus Pentafluoride?
Phosphorus pentafluoride is a type of phosphorus halide. This means it is a compound where phosphorus is bonded to a halogen element, in this case, fluorine. It has a unique shape called a trigonal bipyramidal shape. Imagine two pyramids joined at their bases!
How is it Made?
This gas can be created when two other chemicals react together. One of these is OF2, and the other is phosphorus. When they meet, they combine to form phosphorus pentafluoride.
Cool Facts About PF5
- It is a very light gas. Its density is about 5.527 grams per cubic centimeter.
- It gets very, very cold before it turns into a liquid. Its melting point is around -93.78 degrees Celsius.
- It boils and turns back into a gas at about -84.6 degrees Celsius.
- It is not flammable, which means it will not catch fire easily.
Related Chemicals
Phosphorus pentafluoride has some chemical relatives. These include other compounds made from phosphorus and halogens, like Phosphorus pentachloride or Phosphorus pentabromide. There are also similar compounds where other elements like Arsenic pentafluoride or Antimony pentafluoride are bonded to fluorine.
See also

- Pentafluoruro de fósforo para niños (in Spanish)
 | Precious Adams |
 | Lauren Anderson |
 | Janet Collins |

