Antimony pentafluoride facts for kids
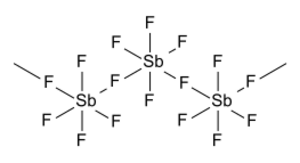
Antimony pentafluoride is a special chemical. It is also known as antimony(V) fluoride. Its chemical formula is SbF5. This means it has one antimony atom connected to five fluorine atoms. The antimony part of this chemical has a +5 charge, which makes it very reactive.
What it is Like
Antimony pentafluoride looks like a thick, clear liquid. It is a very strong chemical that reacts easily with many other things. We call it a powerful "oxidizing agent." This means it can take electrons from other chemicals. It can even make oxygen react in a way that oxygen usually doesn't. It can also create very strong acids when mixed with hydrofluoric acid. This chemical was important in early experiments to make fluorine.
How it is Made
Scientists make antimony pentafluoride by mixing antimony pentoxide or antimony pentachloride with hydrofluoric acid. It can also be made by reacting antimony trifluoride with fluorine.
What it is Used For
Antimony pentafluoride is mainly used to create extremely strong acids. These acids are called "superacids."
Staying Safe
Antimony pentafluoride reacts with most other chemicals. It is extremely dangerous and can cause severe damage if it touches skin or other materials. Always handle it with extreme care.
Related pages
See also
 In Spanish: Pentafluoruro de antimonio para niños
In Spanish: Pentafluoruro de antimonio para niños
 | Roy Wilkins |
 | John Lewis |
 | Linda Carol Brown |