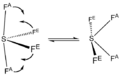Sulfur tetrafluoride facts for kids
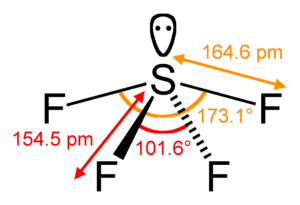
Sulfur tetrafluoride is a special kind of chemical compound. Its chemical formula is SF4. This means each molecule has one sulfur atom and four fluorine atoms. Sulfur tetrafluoride is also sometimes called sulfur(IV) fluoride.
Contents
What is Sulfur Tetrafluoride Like?
Sulfur tetrafluoride is a gas that has no color. It is a very strong chemical. This gas is dangerous because it is poisonous and can damage things it touches. If sulfur tetrafluoride touches water, it reacts strongly and creates another harmful chemical called hydrogen fluoride.
How is Sulfur Tetrafluoride Made?
Scientists can make sulfur tetrafluoride in a lab. They do this by mixing and reacting three other chemicals:
Sometimes, chlorine is not used in the process. However, if chlorine is left out, other unwanted chemicals can form. These extra chemicals then need to be carefully removed.
What is Sulfur Tetrafluoride Used For?
Sulfur tetrafluoride is mainly used in organic chemistry. It helps create special organic molecules that contain fluoride atoms. These molecules are important for making other useful substances. However, sulfur tetrafluoride is not used very often because it is so reactive and dangerous to handle.
Related Pages
Images for kids
See also
 In Spanish: Tetrafluoruro de azufre para niños
In Spanish: Tetrafluoruro de azufre para niños
 | Jessica Watkins |
 | Robert Henry Lawrence Jr. |
 | Mae Jemison |
 | Sian Proctor |
 | Guion Bluford |