Oxide facts for kids

An oxide is a special type of chemical compound that always contains at least one oxygen atom combined with another element. Think of it like a team where oxygen is always one of the players! You might not realize it, but oxides are super common. In fact, most of the Earth's crust, which is the outer layer we live on, is made up of different oxides. Oxides often form when other elements react with oxygen, especially the oxygen found in the air around us. This process is called oxidation.
Common Oxides Around Us
Oxides are everywhere! Here are some examples you might know:
- Water (H2O): This is hydrogen oxide, and it's essential for all life.
- Iron(III) oxide (Fe2O3): You probably know this as Rust. It forms when iron reacts with oxygen and moisture.
- Aluminium oxide (Al2O3): This strong material is found in many rocks and is used to make things like ceramics.
- Carbon dioxide (CO2): A gas we breathe out and plants use for photosynthesis.
- Carbon monoxide (CO): A dangerous gas that can be produced when things burn without enough oxygen.
- Silicon dioxide (SiO2): This is the main ingredient in sand and quartz.
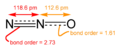
- Nitrous oxide (N2O): Also known as "laughing gas," it's used in medicine and as a propellant.
- Magnesium oxide (MgO): Used in medicines and as a building material.
- Sulfur dioxide (SO2) and Sulfur trioxide (SO3): These gases can cause acid rain.
How Oxides Form
Oxides form when an element reacts with oxygen. This reaction can happen in different ways:
- Burning: When things burn, like wood or natural gas, they combine with oxygen in the air to form oxides. For example, burning wood creates carbon dioxide and water (hydrogen oxide).
- Rusting: When metals like iron are exposed to air and water, they slowly react with oxygen to form metal oxides, like rust.
- Natural Processes: Many rocks and minerals in the Earth's crust are oxides that formed over millions of years through natural geological processes.
Importance of Oxides
Oxides play a huge role in our world:
- Life: Water (hydrogen oxide) is vital for all living things. Carbon dioxide is crucial for plants to grow.
- Industry: Many oxides are used to make important materials. For example, aluminium oxide is used to make strong ceramics and abrasives. Silicon dioxide is used to make glass and computer chips.
- Earth's Structure: The Earth's crust is mostly made of oxides, including silicon dioxide, aluminium oxide, and iron oxides, which form many common rocks and minerals.
Images for kids
-
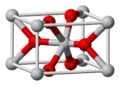
This image shows the tiny building block, called a unit cell, of a mineral called rutile, which is a form of titanium dioxide. The grey balls are titanium atoms, and the red balls are oxygen atoms.
-
Nitrous oxide ("laughing gas") is a gas that can trap heat in the atmosphere, making it a greenhouse gas. It is produced by tiny bacteria in the soil.
See also
 In Spanish: Óxido para niños
In Spanish: Óxido para niños
 | Sharif Bey |
 | Hale Woodruff |
 | Richmond Barthé |
 | Purvis Young |