Island of stability facts for kids
The chemical elements that are heavier than uranium are all radioactive. This means they are not stable and will change into other elements over time. Most of these superheavy elements exist for only a very short time, sometimes just seconds or even less! However, scientists have a cool idea called the Island of Stability. This idea suggests that some superheavy elements might actually be much more stable and last longer than their neighbors.
Contents
What is the Island of Stability?
The idea of the Island of Stability comes from how scientists think the inside of an atomic nucleus is built. Imagine an atom's nucleus is like an onion, with different layers or "shells." These shells are filled with tiny particles called protons and neutrons.
How Nuclear Shells Work
Just like electrons in an atom fill up electron shells, protons and neutrons in the nucleus also fill up their own shells. When a shell is completely full, the nucleus becomes extra stable. This is similar to how a full set of building blocks makes a structure stronger. When the number of protons and neutrons perfectly fills these shells, the nucleus holds together more tightly. This makes the element last longer.
Scientists call the special numbers of protons or neutrons that fill a shell "magic numbers." If an element has a magic number of both protons and neutrons, it's called "doubly magic" and is expected to be super stable! For example, lead-208 is a very stable element because it's doubly magic.
Predicted Magic Numbers
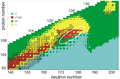
For spherical (round) nuclei, scientists predict that 184 neutrons could be a magic number. For protons, possible magic numbers are 114, 120, and 126. This means that elements like ununquadium-298 (114 protons, 184 neutrons), unbinilium-304 (120 protons, 184 neutrons), and unbihexium-310 (126 protons, 184 neutrons) could be on the Island of Stability.
Unbihexium-310 is especially interesting because it would have 126 protons and 184 neutrons, making it "doubly magic." This means it could have a very long half-life, possibly lasting for minutes or even longer!
Deformed Nuclei and New Magic Numbers
Recent studies show that very large nuclei might not be perfectly round. Instead, they can be "deformed" or squashed. This changes where the magic numbers are. For example, Hassium-270 is now thought to be a doubly magic deformed nucleus, with magic numbers of 108 protons and 162 neutrons. However, it still has a very short half-life of only 3.6 seconds.
Why is the Island of Stability Important?
Scientists are trying to create these superheavy elements in labs. They have made some elements with enough protons to be on the island, but they don't have enough neutrons yet to make them truly stable.
If scientists can create elements on the Island of Stability, these elements might have very unusual chemical properties. If they last long enough, they could even be useful for things like making special targets for particle accelerators or as neutron sources.
Images for kids
-
This diagram shows how superheavy nuclei are predicted to break down. The elements with black outlines have been observed. Some elements might break down by spontaneous fission (SF), where they split into smaller pieces. Others might undergo alpha decay (α), where they release an alpha particle. Some might even have beta decay (β) or electron capture (EC), where a proton changes into a neutron or vice versa.
See also
 In Spanish: Isla de estabilidad para niños
In Spanish: Isla de estabilidad para niños
 | George Robert Carruthers |
 | Patricia Bath |
 | Jan Ernst Matzeliger |
 | Alexander Miles |