Spontaneous fission facts for kids
Spontaneous fission (SF) is a special type of radioactive decay. In this process, a heavy atomic nucleus splits into two or more smaller nuclei. It's different from induced fission, where a particle like a neutron hits the nucleus to make it split. Spontaneous fission happens all by itself, without any outside help. It's a natural, random process.
This kind of splitting is very common for superheavy elements. These are elements with a lot of protons and neutrons. The heavier an element is, the less stable its nucleus usually becomes. This means spontaneous fission sets a limit on how many protons and neutrons an atom can have and still exist for a noticeable time. While heavier atoms can be made, they quickly break apart into more stable ones. Because of this, spontaneous fission isn't usually seen in nature, except for tiny amounts from very old radioactive elements.
The time it takes for half of a sample to split (called a half-life) can be very short, like 4.1 microseconds for Template:OptionalLink. Or it can be incredibly long, even longer than the age of the universe, like for Template:OptionalLink.
Contents
How We Discovered Spontaneous Fission
Scientists first learned about induced fission in 1938. This was thanks to Otto Hahn and Fritz Strassmann. After their discovery, two Soviet physicists, Georgy Flyorov and Konstantin Petrzhak, started their own experiments. They were studying how neutrons affected uranium atoms.
During their tests, they noticed something strange. Their equipment detected bits of atoms splitting even when there were no neutrons around to cause it. They even moved their equipment 60 meters underground into the Dinamo subway station in Moscow. They hoped this would block out cosmic rays, which are tiny particles from space. But the splitting still happened!
Since no one knew of any other way atoms could split without being hit by something, this was a big surprise. The only explanation was that the uranium nuclei were splitting on their own. This is how spontaneous fission was discovered.
How Spontaneous Fission Works
Spontaneous fission happens because of a fight between two powerful forces inside an atom's nucleus. One force is the strong nuclear force. This force tries to hold the protons and neutrons together. It's like a very strong glue. The other force is coulombic repulsion. This force pushes protons away from each other because they all have a positive electric charge.
In light atoms, the strong nuclear force easily wins, keeping the nucleus stable. But in very heavy atoms, there are so many protons packed together. Their repulsion becomes very strong. It can start to overpower the strong nuclear force. When this happens, the nucleus can become more stable if it splits into two smaller pieces. Each smaller piece has less proton repulsion.
Spontaneous fission is usually a slow process. The nucleus can't just jump into two pieces. Instead, it has to "tunnel" through an energy barrier. Think of it like a ball on one side of a hill. To get to the other side (the split state), it needs to go over the hill. But in the quantum world, sometimes the ball can magically appear on the other side without going over! This is called quantum tunneling. The chance of this happening depends on how high the "hill" (energy barrier) is. The higher the barrier, the less likely it is to happen.
This splitting can happen for atoms with 93 or more protons and neutrons. But we mostly see it happen for atoms with 232 or more protons and neutrons. The higher the number of protons, the lower the energy barrier usually is.
Scientists use a "fissility parameter" to describe how likely an atom is to split. This value compares the pushing-apart force (Coulomb energy) to the holding-together force (surface energy). For very heavy nuclei, this parameter gets very high. This means the nucleus becomes very unstable and is likely to split.
Sometimes, the way protons and neutrons are arranged in shells inside the nucleus can also affect how long an atom lasts before splitting. Atoms with an odd number of protons or neutrons tend to split much slower than those with even numbers.
Scientists believe that atoms with about 300 protons and neutrons might have no barrier to fission at all. This means they would split almost instantly. However, there might be a special "island of stability" for atoms around 114 protons and 184 neutrons. These atoms might be more stable than others around them.
Scientists use models to try and understand fission. One simple idea is the "liquid-drop model". It treats the nucleus like a tiny drop of liquid. This model helps explain some things, but it's not perfect because it doesn't fully account for the quantum nature of atoms.
In this liquid-drop idea, the nucleus tries to stay round, like a water drop. But the protons pushing each other away try to stretch the nucleus into an oval shape. If the nucleus stretches too much, especially for large nuclei, a thin "neck" might form. This neck can then break, splitting the nucleus into two fragments. Even if the neck doesn't fully break, quantum tunneling means there's always a chance it will split. After they split, the two new pieces are both positively charged. They push each other away very fast, gaining a lot of energy.
Sometimes, a nucleus can get into a special excited state called a shape isomer before it splits. In this state, the nucleus is already stretched out more than usual. From this state, it's much easier for the nucleus to tunnel through the barrier and split. This means atoms in a shape isomer state will split much faster.
What Comes Out When Atoms Split
When a nucleus splits, the new pieces (called fission fragments) are usually rich in neutrons and have a lot of extra energy. They are also in an "excited state." This means they quickly get rid of their extra energy.
Right after splitting (within a tiny fraction of a second), the fragments usually shoot out neutrons. This is the main way they lose energy at first. After that, they start releasing energy as gamma rays (a type of light). Finally, they might release X-rays.
The new atoms created by this process are often unstable themselves. They might undergo further decay, like beta-decay, and release more photons or neutrons. These are called 'delayed emissions' and can happen over a long time, from tiny fractions of a second to many years.
Because there are so many ways a nucleus can split, the final products can vary a lot. However, the fragments usually have two different sizes. One piece is usually around 95 protons and neutrons, and the other is around 140. Scientists don't fully understand why spontaneous fission doesn't usually create two equal-sized pieces.
Very rarely (about 0.3% of the time), three or more fragments might be created. These extra pieces are usually small, like alpha-particles (helium nuclei), but can sometimes be as big as oxygen nuclei.
When an atom splits, it releases a huge amount of energy, about 200 MeV. Most of this energy is seen as the kinetic energy (energy of motion) of the fission fragments. The lighter fragment usually gets more of this energy.
The number of neutrons released when an atom splits isn't always the same. It varies, but the average number of neutrons is consistent. These "prompt neutrons" are released with a range of energies, usually between 0.5 and 1 MeV, with an average of 2 MeV.
Besides kinetic energy and neutrons, about 8 MeV of energy is released as prompt gamma rays. Later, beta decay and delayed gamma rays contribute another 19 MeV and 7 MeV respectively. Less than 1% of the neutrons released are delayed neutrons.
Uses of Spontaneous Fission
The most common use for spontaneous fission is as a source of neutrons. These neutrons can be used for many things, like:
- Neutron imaging: This is like X-rays, but uses neutrons to see inside materials.
- Nuclear reactions: The neutrons can start other nuclear reactions. This includes causing induced fission in things like nuclear reactors (for power) and nuclear weapons.
In certain crystals that contain a lot of uranium, the fission products from spontaneous fission can leave tiny damage trails as they move through the crystal. Scientists can count these trails, called fission tracks, to figure out how old the crystal sample is. This method is called fission track dating.
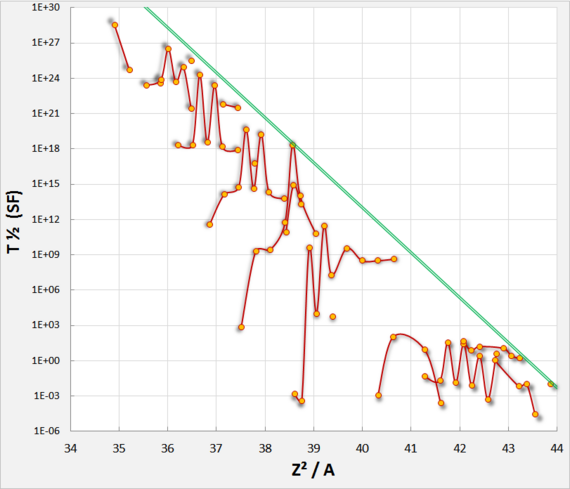
Spontaneous Fission Rates
| Nuclide | Half-life (years) |
Fission branching ratio (% of decays) |
Neutrons per | Spontaneous half-life (years) |
Z2A | |
|---|---|---|---|---|---|---|
| Fission | Gram-sec | |||||
| Template:OptionalLink | 7.04·108 | 2.0·10−7 | 1.86 | 0.0003 | 3.5·1017 | 36.0 |
| Template:OptionalLink | 4.47·109 | 5.4·10−5 | 2.07 | 0.0136 | 8.4·1015 | 35.6 |
| Template:OptionalLink | 24100 | 4.4·10−10 | 2.16 | 0.022 | 5.5·1015 | 37.0 |
| Template:OptionalLink | 6569 | 5.0·10−6 | 2.21 | 920 | 1.16·1011 | 36.8 |
| Template:OptionalLink | 8300 | ~74 | 3.31 | 1.6·1010 | 1.12·104 | 36.9 |
| Template:OptionalLink | 2.6468 | 3.09 | 3.73 | 2.3·1012 | 85.7 | 38.1 |
See also
- Nuclear fission
- Natural nuclear fission reactor
- Alpha decay
- Cluster decay
 | Janet Taylor Pickett |
 | Synthia Saint James |
 | Howardena Pindell |
 | Faith Ringgold |