Ununennium facts for kids
| Ununennium | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | |||||||||||||||||||||
| Alternative names | element 119, eka-francium | ||||||||||||||||||||
| Mass number | 315 (predicted) | ||||||||||||||||||||
| Ununennium in the periodic table | |||||||||||||||||||||
|
|||||||||||||||||||||
| Atomic number (Z) | 119 | ||||||||||||||||||||
| Group | group 1: hydrogen and alkali metals | ||||||||||||||||||||
| Period | period 8 | ||||||||||||||||||||
| Block | s | ||||||||||||||||||||
| Electron configuration | [Og] 8s1 (predicted) | ||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 32, 32, 18, 8, 1 (predicted) | ||||||||||||||||||||
| Physical properties | |||||||||||||||||||||
| Phase at STP | unknown (could be solid or liquid) | ||||||||||||||||||||
| Melting point | 273–303 K (0–30 °C, 32–86 °F) (predicted) | ||||||||||||||||||||
| Boiling point | 903 K (630 °C, 1166 °F) (predicted) | ||||||||||||||||||||
| Density (near r.t.) | 3 g/cm3 (predicted) | ||||||||||||||||||||
| Heat of fusion | 2.01–2.05 kJ/mol (extrapolated) | ||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||
| Oxidation states | (+1), (+3) (predicted) | ||||||||||||||||||||
| Electronegativity | Pauling scale: 0.86 (predicted) | ||||||||||||||||||||
| Ionization energies |
|
||||||||||||||||||||
| Atomic radius | empirical: 240 pm (predicted) | ||||||||||||||||||||
| Covalent radius | 263–281 pm (extrapolated) | ||||||||||||||||||||
| Other properties | |||||||||||||||||||||
| Crystal structure | body-centered cubic (bcc)
(extrapolated) |
||||||||||||||||||||
| CAS Number | 54846-86-5 | ||||||||||||||||||||
| History | |||||||||||||||||||||
| Naming | IUPAC systematic element name | ||||||||||||||||||||
| Main isotopes of ununennium | |||||||||||||||||||||
|
|||||||||||||||||||||
Ununennium, also known as eka-francium or element 119, is a chemical element that scientists believe might exist. Its temporary symbol is Uue, and its atomic number is 119. The name "Ununennium" and symbol "Uue" are used until the element is officially discovered, confirmed, and given a permanent name. In the periodic table of the elements, Ununennium is expected to be an alkali metal, like lithium and sodium. It would be the first element in the eighth row of the periodic table. It is the lightest element that scientists have not yet created.
Contents
What is Ununennium?
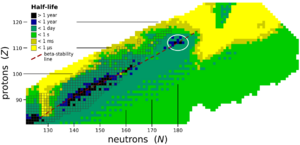
Superheavy elements are those with atomic numbers greater than 103. They are not found naturally on Earth. Scientists create them in laboratories by smashing smaller atoms together. These elements are usually very unstable and break down quickly. Ununennium, if created, would be one of these superheavy elements.
How Scientists Try to Make New Elements
Past Attempts to Create Element 119
Scientists have been trying to make element 119 for many years. Elements 114 to 118 were made in Russia by hitting heavy atoms with calcium-48. To make element 119 this way, scientists would need a special target atom called einsteinium. However, only tiny amounts of einsteinium are available. In 1985, an early attempt to make element 119 using einsteinium did not succeed. Making new superheavy elements is very hard. The chances of success are very small. Also, these new elements are expected to break down almost instantly. From April to September 2012, scientists in Germany tried to make element 119. They hit berkelium-249 with titanium-50. This was thought to be the best way at the time. They also looked for element 120 at the same time. Despite their efforts, neither element 119 nor element 120 was found.
Current Experiments
Scientists at RIKEN in Japan have been trying to make element 119 since January 2018. They are using targets made of curium-248 and hitting them with a beam of vanadium-51. Curium was chosen because it is easier to get than other heavy targets. The RIKEN team has been running this experiment continuously, 24 hours a day, 7 days a week, as of August 2024. They hope to see the first sign of element 119 soon. If they succeed, the new ununennium atoms would quickly break down into known elements like moscovium. This would help confirm their discovery.
Future Plans for Element 119
The Joint Institute for Nuclear Research (JINR) in Russia is starting an attempt to make element 119 in 2026. In late 2023, the JINR reported the first successful creation of a superheavy element using a projectile heavier than calcium-48. They made a new isotope of livermorium (element 116) by hitting uranium-238 with chromium-54. This success gives them hope for making element 119. They plan to hit americium-243 with chromium-54. Another team in China, at the Heavy Ion Research Facility in Lanzhou (HIRFL), also plans to try the same reaction.
Naming New Elements
When a new element is discovered, it first gets a temporary name. For element 119, this temporary name is "Ununennium," and its symbol is "Uue." This name comes from a system that uses Latin and Greek numbers. Before this system, scientists sometimes used names like "eka-francium," meaning "below francium," to guess its properties. Once an element's discovery is confirmed, the scientists who found it get to suggest a permanent name.
What We Think Ununennium Would Be Like
Scientists use powerful computers and physics rules to predict what new elements might be like.
How Stable Would It Be?
Heavy elements tend to be very unstable. They break down quickly. However, scientists believe there's a special region called the "island of stability." This is where some superheavy elements might last a bit longer than others. Element 119 is expected to be very unstable, breaking down in tiny fractions of a second. This makes it very hard to detect. Scientists are always refining their models to better understand where this "island of stability" truly lies.
Its Atomic and Physical Traits
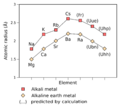
Ununennium is predicted to be an alkali metal, just like lithium, sodium, and potassium. These elements usually have one electron in their outer shell, which they easily lose in chemical reactions. However, because Ununennium is so heavy, its electrons move incredibly fast. This causes "relativistic effects," which means its properties might be a bit different from what we expect. For example, Ununennium might be less reactive than francium, its lighter family member. It could behave more like potassium or rubidium. Scientists predict that Ununennium would need more energy to lose its first electron compared to other alkali metals. It might also be better at attracting electrons. Its atomic size is predicted to be similar to rubidium. Scientists think Ununennium might be a liquid at room temperature, with a melting point between 0°C and 30°C. Its boiling point could be around 630°C. Its density is expected to be higher than francium or caesium.
How It Might React Chemically
Ununennium's chemistry should be similar to other alkali metals. But due to those "relativistic effects," it might act more like rubidium or potassium than francium. Interestingly, Ununennium might be able to form compounds where it shares three or even five electrons. This is very unusual for an alkali metal, as they typically only share one. This is because some of its inner electrons might become more involved in bonding. Ununennium is also expected to form strong bonds by sharing electrons, especially with elements like fluorine. This is different from how lighter alkali metals usually bond. Scientists predict that Ununennium would not stick as strongly to surfaces like gold, platinum, or silver compared to other alkali metals. This information helps them design experiments to study it if they ever create it.
Images for kids
See also
 In Spanish: Ununennio para niños
In Spanish: Ununennio para niños
 | Kyle Baker |
 | Joseph Yoakum |
 | Laura Wheeler Waring |
 | Henry Ossawa Tanner |