Nuclear chemistry facts for kids
Nuclear chemistry is a special part of chemistry that studies radioactivity, nuclear processes, and changes happening inside the center (nucleus) of atoms. It looks at how atoms with unstable nuclei behave, how they change into other atoms, and how we can use these processes. It's also about the chemistry of radioactive elements and the chemical reactions that happen around equipment like nuclear reactors.
Nuclear chemistry is a vital field that helps us understand the fundamental processes happening within atomic nuclei, harness nuclear energy, develop life-saving medical treatments, and manage radioactive materials safely. It's a science with a rich history and important applications for our future.
Contents
Understanding the Basics: Atoms and Radioactivity
You probably know that everything around us is made of tiny building blocks called atoms. Each atom has a nucleus at its center, made up of protons and neutrons, and electrons orbiting around it.
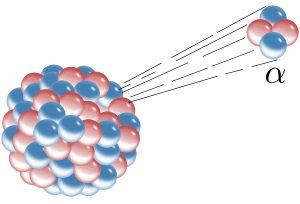
In nuclear chemistry, we focus on the nucleus. Sometimes, the nucleus of an atom is unstable. This means it has too much energy or an unbalanced number of protons and neutrons. To become stable, the nucleus releases energy and tiny particles. This process is called radioactivity, and the atoms that do this are called radioactive isotopes or radionuclides.
When a radioactive nucleus releases particles or energy, it often changes into a different atom or a different form of the same atom. This change is called nuclear transformation or radioactive decay. Nuclear chemistry studies these transformations and the properties of these special atoms.
History
The story of nuclear chemistry began not too long ago, in the late 1800s.
It started when Wilhelm Röntgen discovered X-rays, a type of energy that could pass through things and show bones inside the body. This got many scientists interested in invisible rays.
Henri Becquerel in France was studying materials that glowed after being exposed to light (like phosphorescence). He was working with uranium and photographic plates. One day, he put a piece of uranium on a photographic plate wrapped in dark paper, even though there was no light. Later, he found the plate was blackened, just as if it had been exposed to light! This meant the uranium was giving off its own invisible rays, which he called "uranic rays." This was the discovery of radioactivity!
Inspired by Becquerel, Marie Skłodowska-Curie and her husband Pierre Curie in Paris started studying these rays. They examined different materials and found that some ores containing uranium were even more radioactive than pure uranium. They realized there must be other, more radioactive elements in the ore. Using chemical methods to separate the ore into different parts, they carefully measured the radioactivity of each part. This led them to discover two new radioactive elements: polonium (named after Marie's home country, Poland) and radium. Their work was incredibly important and showed the power of combining chemistry with the study of radioactivity.
Around 1901, people started noticing that strong radiation could affect living things. Henri Becquerel himself got a burn from carrying a sample of radium in his pocket! This showed that radiation had biological effects and led to scientists studying how radiation interacts with living things, which eventually helped develop medical treatments like cancer therapy.
Ernest Rutherford, working in Canada and England, made huge steps in understanding radioactive decay. He showed that radioactive substances decay at a specific rate, introducing the idea of a "half-life" – the time it takes for half of the radioactive atoms in a sample to decay. He also named the different types of radiation: alpha, beta, and gamma rays. Rutherford also famously conducted experiments (like the gold foil experiment done by his students) that showed that atoms have a tiny, dense, positively charged center – the nucleus! This changed how we pictured the atom.
Marie Curie's daughter, Irène Joliot-Curie, and her husband, Frédéric Joliot-Curie, made a groundbreaking discovery: they created artificial radioactivity! They bombarded stable atoms with particles and turned them into new, radioactive isotopes that didn't exist naturally.
Otto Hahn was another key figure. He developed methods to study tiny amounts of radioactive materials. In 1938, Hahn, Lise Meitner, and Fritz Strassmann made one of the most important discoveries: nuclear fission. They found that when a neutron hits a uranium nucleus, it can split into smaller nuclei, releasing a huge amount of energy. This discovery became the basis for nuclear power and nuclear weapons. Otto Hahn is often called the "father of nuclear chemistry."
What Does Nuclear Chemistry Study Today?
Nuclear chemistry is a broad field with several important areas:
- Radiochemistry: This is the study of radioactive materials themselves. Radiochemists work with radioactive isotopes to understand their chemical properties and reactions. They might use radioactive isotopes as tracers to follow chemical processes or study the chemistry of elements like uranium, plutonium, and other elements created in nuclear reactions.
- Radiation Chemistry: This area studies the chemical effects that radiation has on matter. It's different from radiochemistry because the material being changed by radiation doesn't have to be radioactive itself. For example, radiation can break apart water molecules into other substances. Understanding these effects is important for safety and for using radiation in processes like changing materials or sterilizing equipment.
- Chemistry for Nuclear Power: Nuclear chemistry is essential for everything related to nuclear power plants. This includes preparing the fuel (like uranium) from ores, understanding the chemistry of the coolants used in reactors, dealing with used nuclear fuel, which is still radioactive, treating and storing radioactive waste safely, monitoring for any release of radioactive materials, studying how materials behave inside a reactor under extreme conditions.
- Studying Nuclear Reactions: Nuclear chemists and physicists work together to study nuclear reactions like fission (splitting atoms) and fusion (joining atoms). They use chemical methods to identify and separate the products of these reactions. This is how scientists first found evidence for nuclear fission and how they are trying to create new, superheavy elements that don't exist naturally.
- The Nuclear Fuel Cycle: This covers all the steps involved in using nuclear fuel, from mining the uranium ore to making the fuel, using it in a reactor, and finally managing the used fuel. Nuclear chemistry is involved in every step, including processes like reprocessing, where useful materials like uranium and plutonium are separated from used fuel so they can potentially be reused. This involves complex chemical separation techniques, like the PUREX process, which uses special chemicals to extract desired elements. Scientists are also researching newer methods like UREX, TRUEX, DIAMEX, and SANEX to improve efficiency and safety in managing nuclear materials.
- How Radioactive Materials Interact with Surfaces: Nuclear chemists study how radioactive substances stick to or react with different surfaces. This is important for designing safe containers for nuclear waste and understanding how radioactive materials might move in the environment or within a nuclear plant during normal operation or in the unlikely event of an accident. For example, certain radioactive substances like technetium can form layers on steel that help prevent corrosion.
Beyond the Nucleus: Spinout Areas
Some techniques and ideas that started in nuclear chemistry and physics are now used widely in many other fields:
- Kinetics: By replacing a normal atom (like hydrogen) with its heavier isotope (like deuterium), scientists can see how the speed of a chemical reaction changes. This "kinetic isotope effect" helps chemists figure out exactly how a reaction happens, step by step.
- Geology: Scientists use isotopes found in rocks (formed by cosmic rays or radioactive decay) to figure out the age of rocks and understand Earth's history.
- Biology and Medicine: Isotopes can be used as "tracers" to follow how substances move and change inside living organisms. For example, radioactive isotopes are used in medical imaging (like PET scans) and cancer treatment (radiotherapy). Stable isotopes are used in studies to understand metabolism or track the origin of materials. NMR (Nuclear Magnetic Resonance), which uses the magnetic properties of atomic nuclei, is a standard tool in chemistry to identify molecules and is also used in medicine for MRI scans (Magnetic Resonance Imaging) to see inside the body without using harmful radiation.
- Forensic Science: Isotope analysis can sometimes help trace the origin of materials like bullets or determine a person's diet from hair samples.
See also
 In Spanish: Química nuclear para niños
In Spanish: Química nuclear para niños
- Important publications in nuclear chemistry
- Nuclear physics
- Nuclear spectroscopy
 | Sharif Bey |
 | Hale Woodruff |
 | Richmond Barthé |
 | Purvis Young |

