Deuterium facts for kids
Deuterium is a special type of hydrogen. Hydrogen is the very first element on the periodic table. Deuterium is different from regular hydrogen because it has one proton and one neutron in its center, called the nucleus. Regular hydrogen only has one proton and no neutrons. Another special type of hydrogen, called tritium, has one proton and two neutrons. Scientists often use the chemical symbol D for deuterium, but sometimes you might see 2H.
Contents
What is Deuterium?
Deuterium is known as an isotope of hydrogen. An isotope is like a cousin of an element. It's the same element, but its atoms have a different number of neutrons. This makes them a bit heavier.
Deuterium is a stable isotope. This means it does not break down over time. It is found naturally in small amounts. About one out of every 6,420 hydrogen atoms on Earth is a deuterium atom.
Deuterium and Heavy Water
When two deuterium atoms join with one oxygen atom, they form something called "heavy water." Normal water is H2O, meaning two hydrogen atoms and one oxygen atom. Heavy water is D2O.
Heavy water is heavier than regular water. This is because deuterium atoms have an extra neutron. This extra neutron adds more mass to the water molecule.
Uses of Heavy Water
Heavy water has some important uses:
- Nuclear Reactors: It is used in some nuclear power plants. It helps to slow down tiny particles called neutrons. This process is important for controlling the power of the reactor.
- Scientific Research: Scientists use heavy water in special studies. For example, it helps them understand how molecules are built. It can also be used to study how water moves through plants and animals.
Who Discovered Deuterium?
Deuterium was discovered in 1931. An American chemist named Harold Urey found it. He won the Nobel Prize in Chemistry in 1934 for his discovery.
Images for kids
-
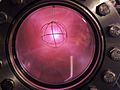
Deuterium discharge tube
-
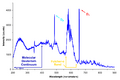
Emission spectrum of an ultraviolet deuterium arc lamp
-
Harold Urey, deuterium's discoverer
-
The "Sausage" device casing of the Ivy Mike H bomb, attached to instrumentation and cryogenic equipment. The 20-ft-tall bomb held a cryogenic Dewar flask with room for 160 kg of liquid deuterium.
See also
 In Spanish: Deuterio para niños
In Spanish: Deuterio para niños