Vacuum flask facts for kids


A vacuum flask is a special container that keeps things hot or cold for a long time. You might know it as a Dewar flask or a thermos. It was invented by Sir James Dewar in 1892.
This flask has two containers, one inside the other. The space between them has almost no air, creating a near-vacuum. This vacuum is super important because it stops heat from moving in or out easily. It reduces heat transfer by conduction (heat moving through solid materials) and convection (heat moving through liquids or gases). If you put cold liquids in it, the vacuum also stops water from forming on the outside (condensation).
People use vacuum flasks at home to keep drinks hot or cold. They are also great for keeping cooked food warm. Sometimes, they are even used for thermal cooking, where food cooks slowly inside the flask. Industries also use them for many different jobs.
Contents
How the Vacuum Flask Was Invented
The vacuum flask was created by a Scottish scientist named James Dewar in 1892. He was studying very cold temperatures, a field called cryogenics. He needed a way to keep his experiments at a steady temperature. So, he made a brass container and put it inside another one. He then removed the air between them, making a partial vacuum. This helped keep the temperature inside stable.
Dewar chose not to patent his invention. This meant other people could use his idea freely. Soon, others started making these flasks with new materials like glass and aluminium. The vacuum flask became a very important tool for science and a common item in homes.
In 1904, two German glassblowers, Reinhold Burger and Albert Aschenbrenner, saw the everyday potential of Dewar's design. They realized it could keep drinks hot or cold for everyday use. They made a stronger version of the flask. They named their commercial product Thermos and got the rights to the name.
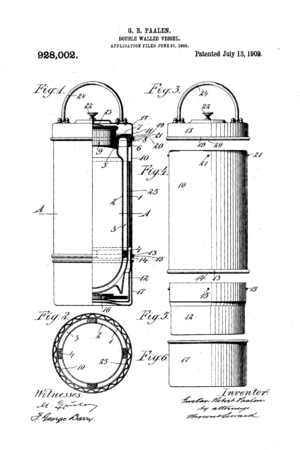
Later, a Viennese inventor named Gustav Robert Paalen improved the Thermos bottle even more. He designed different types for home use and patented them. Companies in the United States, Canada, and the UK bought licenses to make and sell them. The American Thermos Bottle Company started making them in large numbers in Norwich, CT. This made them cheaper and available to many more families. Over time, these companies made flasks of different sizes, shapes, and materials. People mostly used them to carry coffee or liquids for camping trips.
The word "thermos" eventually became a common word for any vacuum-insulated container. This is called a generic trademark. While Thermos is still a registered trademark in some countries, in the United States, a court decided in 1963 that "thermos" had become a general word for these flasks.
How a Vacuum Flask Works
A vacuum flask is made of two containers, one inside the other, joined at the top. The space between them has most of the air removed, creating a partial-vacuum. This vacuum is key because it greatly slows down heat transfer by conduction (heat moving through materials) and convection (heat moving through liquids or gases).
To further reduce heat transfer, especially by thermal radiation (heat moving as waves), the surfaces facing the vacuum gap are often coated with a shiny material like silver. This helps reflect heat. Most heat escapes through the neck and opening of the flask, as there's no vacuum there.
Vacuum flasks are usually made from metal, borosilicate glass, foam, or plastic. The opening is sealed with a cork or plastic stopper. These flasks are often used as insulated shipping containers for sensitive items.
Sometimes, very large or long vacuum flasks need extra support for the inner container. They use "spacers" between the inner and outer shells. These spacers can let a little heat pass through, slightly reducing how well the flask insulates in those spots.
Some advanced vacuum flasks, like those used in NMR and MRI machines, have two vacuum sections. The inner flask holds liquid helium, and the outer flask holds liquid nitrogen. There's a vacuum between each layer. This design helps prevent the valuable helium from evaporating too quickly.
Other improvements include "vapour-cooled radiation shields" and "vapour-cooled necks." These features also help reduce how much liquid evaporates from the flask.
Vacuum Flasks in Science and Industry
In science labs and factories, vacuum flasks are often used to hold gases that have been turned into liquids. For example, liquid nitrogen (which boils at a super cold 77 K, or -321°F) is kept in these flasks. It's used for things like flash freezing, preparing samples, and other processes where extreme cold is needed.
Larger vacuum flasks store liquids that become gases at normal temperatures, like oxygen and nitrogen. Because heat slowly leaks into the super cold liquid, it causes the liquid to slowly boil off. So, these flasks need a small opening or a special pressure relief valve to let the gas escape. This stops pressure from building up and possibly breaking the flask. The excellent insulation means the liquid stays liquid for a long time without needing a refrigerator.
Vacuum flasks have also been used in very precise electrical devices. For example, they can hold special components like Zener diodes to keep their temperature very stable. This helps make sure the device's output voltage stays incredibly accurate, changing only by tiny amounts.
For instance, a company called Guildline Instruments used a silvered vacuum flask inside foam insulation for their Transvolt, model 9154B. This device was an electrical voltage standard that produced 1.018 volts, and the flask helped keep it stable to within a few parts per million.
The design of the vacuum flask was also very useful for storing certain types of rocket fuel. NASA used them a lot in the fuel tanks of the Saturn rockets in the 1960s and 1970s.
The shape of the Dewar flask has even inspired optical experiments, as its two compartments with a space in between are similar to how light interacts with the eye. Vacuum flasks have also been used in experiments as containers for different chemicals to keep them at a steady temperature.
In the medical world, industrial Dewar flasks are used in devices like the Arktek. This device uses eight one-liter ice blocks to keep vaccines at temperatures below 10 °C. This is important because most vaccines are sensitive to heat and need to be kept cold during transport, which is called a cold chain system.
In the oil and gas industry, Dewar flasks help protect electronic parts in tools used for wireline logging. These tools go deep into wells. By putting sensitive electronics inside a Dewar flask, they can work in much hotter conditions.
Safety Tips for Vacuum Flasks
Vacuum flasks, especially those made of glass, can sometimes be at risk of imploding (collapsing inward). Glass vessels under vacuum can shatter unexpectedly. Small chips, scratches, or cracks can weaken the flask. This is especially true if the temperature inside changes very quickly, like when you add very hot or very cold liquid. It's a good idea to prepare glass Dewar flasks properly before using them, perhaps by letting them slowly adjust to temperature changes.
Glass vacuum flasks are usually placed inside a metal base or covered with mesh, aluminum, or plastic. This helps protect them from damage and contains any broken pieces if they do shatter.
Also, some very large cryogenic storage dewars are pressurized. They can explode if they don't have pressure relief valves to release built-up pressure.
Engineers also need to think about Thermal expansion when designing vacuum flasks. The inner and outer walls are exposed to different temperatures. This means they will expand or shrink at different rates. If not designed carefully, this difference in expansion could cause the flask to break. Special Expansion joints are often used in tube-shaped vacuum flasks to prevent them from breaking and to keep the vacuum sealed.
|
See also
 In Spanish: Termo para niños
In Spanish: Termo para niños