Haber process facts for kids
The Haber process, also called the Haber-Bosch process, is a special way to make a chemical called ammonia. It uses two common gases: nitrogen and hydrogen. This process needs very high temperatures, usually between 400°C and 450°C. It also uses a lot of pressure, about 200 times the normal air pressure. To make the reaction happen faster, a special helper called a catalyst is used. This catalyst is mostly made of iron.
History of the Haber Process
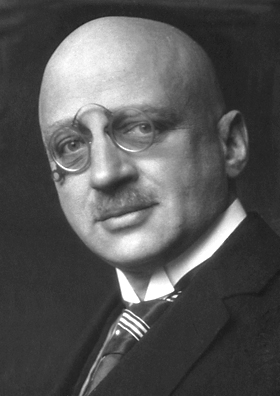
The Haber process is named after a German scientist named Fritz Haber. He was the first person to successfully create ammonia using this method. In 1909, Haber's early process could make about one cup of ammonia every two hours.
Another scientist, Carl Bosch, helped make the Haber process work for big factories. In 1913, a German company called BASF began using it to produce large amounts of ammonia. During World War I, this process was used to make explosives. The Germans kept this a secret until after the war ended.
Fritz Haber won the Nobel Prize in Chemistry in 1918 for his work. Carl Bosch also shared a Nobel Prize in 1931 for his part in developing the process.
Today, the Haber process is still very important. It creates ammonia, which is a key ingredient for fertilizer. This fertilizer helps plants grow better. About 500 million tons (453 billion kilograms) of fertilizer are made each year using this process. This huge amount of fertilizer helps to feed about 40% of the world's population.
How the Process Works
The gases used in the Haber process must be prepared first. After that, ammonia is created using a catalyst. The catalyst is usually magnetite (a type of iron oxide).
The chemical reaction looks like this:
- N2 + 3H2
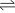 2NH3
2NH3
In each cycle, only about 15% of the nitrogen and hydrogen gases turn into ammonia. However, the gases that don't react are recycled. This means they are sent back into the process to try again. Because of this recycling, almost all (98%) of the nitrogen and hydrogen can eventually be changed into ammonia.
Images for kids
-
Severnside fertilizer plant northwest of Bristol UK
See also
 In Spanish: Proceso de Haber para niños
In Spanish: Proceso de Haber para niños
 | Emma Amos |
 | Edward Mitchell Bannister |
 | Larry D. Alexander |
 | Ernie Barnes |




