Henry Cavendish facts for kids
Quick facts for kids
Henry Cavendish
|
|
|---|---|
 |
|
| Born | 10 October 1731 |
| Died | 24 February 1810 (aged 78) London, England
|
| Citizenship | English |
| Alma mater | Peterhouse, Cambridge |
| Known for | Discovery of hydrogen Measuring the Earth's density (Cavendish experiment) |
| Awards | Copley Medal |
| Scientific career | |
| Fields | Chemistry, physics |
| Institutions | Royal Institution |
Henry Cavendish was a brilliant English scientist. He lived from 1731 to 1810. He was both a chemist and a physicist. Cavendish is famous for discovering hydrogen gas. He called it "inflammable air" because it could burn. He also figured out that this gas forms water when it burns. Later, another scientist named Antoine Lavoisier gave hydrogen its modern name.
Cavendish was a very careful scientist. He was known for his precise experiments. He studied many things. These included the air we breathe and different types of gases. He also explored how water is made. He investigated electricity and how heat works. One of his most famous achievements was measuring the Earth's density. This important experiment is now called the Cavendish experiment.
Contents
Henry Cavendish's Early Life & Education
Henry Cavendish was born on October 10, 1731. His birthplace was Nice, a city in what is now France. His family was quite important and well-known in England. Sadly, his mother passed away when Henry was very young. His father, Lord Charles Cavendish, raised Henry and his younger brother.
From age 11, Henry went to a private school near London. When he was 18, he started studying at Cambridge University. However, he left after three years without getting a degree. This was not unusual for students at that time. After university, he lived with his father in London. He even had his own laboratory there.
Henry's father was also interested in science. He was part of the Royal Society, a famous scientific group. Lord Charles often took Henry to their meetings and dinners. In 1760, Henry became a member of the Royal Society himself. He loved attending these scientific gatherings.
Henry didn't get involved in politics like some of his family. Instead, he focused on science. He helped the Royal Society in many ways. He even led committees to check scientific instruments. His first important scientific paper was published in 1766. It was about different types of gases. He also helped with expeditions, like one looking for the North Pole. Later in life, he became a manager at the Royal Institution. There, he watched and helped with experiments by other scientists, like Humphry Davy.
Discovering Hydrogen and Air's Secrets
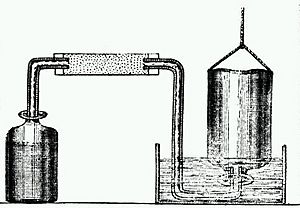
Henry Cavendish was a key figure in a field called "pneumatic chemistry." This was the study of gases. He worked with other famous scientists like Joseph Priestley. Cavendish found a special gas that was very flammable. He called it "Inflammable Air." Today, we know this gas as hydrogen.
Cavendish showed that hydrogen was a unique element. He also correctly figured out that water is made of hydrogen and another gas. He discovered that when hydrogen burns, it creates water. This was a very important finding!
He also studied the air we breathe. In 1777, he found that the air we exhale contains "fixed air." This is what we now call carbon dioxide. He also experimented with different gases. He collected them and measured how much they dissolved in water. He also checked how easily they burned. For his important work, he received the Copley Medal. This is a very prestigious award for scientists.
Uncovering Air's Composition
In 1785, Cavendish did amazing experiments on common air. He mixed hydrogen with ordinary air. Then he used an electric spark to make them explode. He found that air is mostly made of two gases. He called them "phlogisticated air" and "dephlogisticated air." Today, we know these as nitrogen and oxygen.
Cavendish's careful measurements showed that air is about one-fifth oxygen and four-fifths nitrogen. But he noticed something else. After removing all the oxygen and nitrogen, a tiny bubble of gas was always left over. He couldn't explain what this leftover gas was.
About 100 years later, in the 1890s, two other scientists, William Ramsay and Lord Rayleigh, solved the mystery. They discovered a new gas called argon. This argon was the leftover gas Cavendish had found! This showed how incredibly accurate Cavendish's experiments were. He used very precise tools for his time. He always tried to get the most exact results possible.
Understanding Heat
Cavendish also thought deeply about heat. He believed that heat was caused by the movement of tiny particles. This was a very advanced idea for his time. He even developed a theory that included the idea of conservation of energy. This means that energy cannot be created or destroyed. His ideas about heat were far ahead of his time.
Measuring the Earth's Density
One of Henry Cavendish's most famous experiments was to "weigh the Earth." He actually measured the density of our planet. This experiment, published in 1798, is known as the Cavendish experiment.
He used a special device called a torsion balance. This device was originally designed by another scientist, John Michell. Cavendish improved it greatly. His setup had two small lead balls and two much larger lead balls. He wanted to measure the tiny gravitational pull between them.
Cavendish was very careful. He put his equipment in a separate room. He used telescopes to observe it from outside. This prevented air currents or temperature changes from affecting his measurements.
Using this precise method, Cavendish calculated the attraction between the balls. From this, he figured out the Earth's density. He found that the Earth is about 5.48 times denser than water. This was an incredibly accurate result for his time! His measurement is very close to what scientists accept today.
His work also helped later scientists. They used his results to calculate the gravitational constant. This constant is a fundamental number in physics. It describes the strength of gravity.
Exploring Electricity
Cavendish also did many experiments with electricity. He developed a detailed theory about how electricity works. His ideas were based on careful measurements.
He even built an artificial electric fish. This was to show that the shocks from real electric fish were indeed caused by electricity. He published some of his electrical theories in 1771.
Interestingly, much of Cavendish's work on electricity was not known for a long time. He didn't publish many of his findings. About a century after his death, another famous scientist, James Clerk Maxwell, looked through Cavendish's notes. Maxwell found many important discoveries that Cavendish had made. These included ideas about electric potential and capacitance. He also found early versions of laws like Ohm's law and Coulomb's law.
Death and Legacy
Henry Cavendish passed away on February 24, 1810. He was one of the wealthiest people in Britain at the time. He was buried in the church that is now Derby Cathedral.
A Shy but Brilliant Mind
Cavendish was known for being very shy. He often avoided social gatherings. He preferred to speak to only one person at a time, and usually only if it was a man he knew. He was quiet and often seen as eccentric. He even communicated with his female servants using notes!
Despite his shyness, Cavendish was highly respected by other scientists. He almost never missed meetings of the Royal Society Club. His home was more like a laboratory than a comfortable house. He had a huge library and many scientific instruments.
Because he was so private, Cavendish didn't publish all his work. Many of his amazing discoveries were found in his papers years later. These included ideas that other scientists later got credit for. For example, he had insights into Ohm's law and Charles's law of gases.
His legacy lives on in many ways. The famous Cavendish Laboratory at the University of Cambridge is named after him. It was funded by one of his relatives. People in his neighborhood used to point to his laboratory and say it was "where the world was weighed." This shows how famous his Earth density experiment became.
Selected Writings
See also
 In Spanish: Henry Cavendish para niños
In Spanish: Henry Cavendish para niños
- Timeline of hydrogen technologies