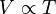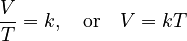Charles's law facts for kids
Charles' law is a science rule about gases. It helps us understand how gases change their size (called volume) when you heat them up or cool them down. Think about a balloon: if you heat it, it gets bigger! If you cool it, it shrinks. Charles' law explains why this happens.
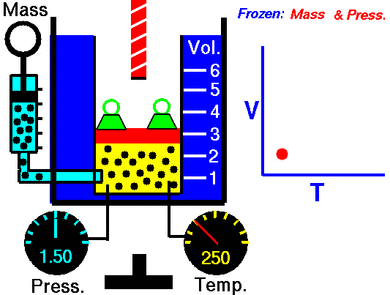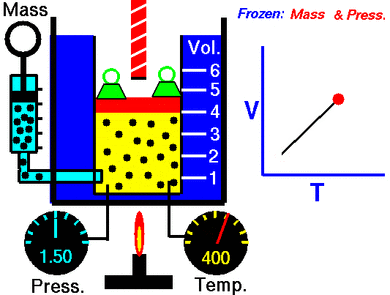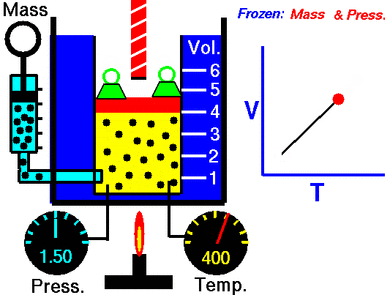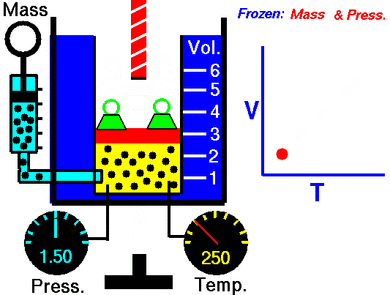
This law says that if you keep the pressure on a gas steady, its volume and its temperature will always go up or down together. This is called a direct proportion.
We can write this idea using a simple math formula:
This means that volume (V) is directly related to temperature (T).
So, if you divide the volume by the temperature, you always get the same number. We call this number 'k', which is a constant.
Here's what the letters mean:
- V is the volume of the gas.
- T is the temperature of the gas. It must be measured in kelvins (a special temperature scale).
- k is a number that stays the same (a constant).
This law shows that when a gas gets hotter, its volume increases. And if it gets colder, its volume decreases. If you want to compare the same gas at two different times or conditions, you can use this formula:
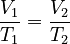
This formula means that the ratio of volume to temperature is always the same for a gas, as long as the pressure doesn't change.
Contents
Who Discovered Charles' Law?
This law is named after a French scientist named Jacques Charles. He first wrote about this idea in his notes way back in the 1780s. But he didn't publish his work.
Later, in 1801, another scientist named John Dalton did experiments that showed all gases expand by the same amount when heated. Then, in 1802, Joseph Louis Gay-Lussac confirmed this discovery. He gave credit to Jacques Charles for his earlier, unpublished work.
Even before Charles, other scientists like Guillaume Amontons and Francis Hauksbee had noticed similar things about gases. But Dalton was the first to show that this rule worked for all gases.
What is Absolute Zero?
Charles' law makes us think about a very cold temperature called absolute zero. If you keep cooling a gas, its volume gets smaller and smaller. Charles' law seems to suggest that at a certain temperature, the gas would have zero volume!
This special temperature is about -273.15 degrees Celsius. Scientists call it absolute zero. At this temperature, gas particles would have almost no energy and would stop moving.
However, Charles' law only works as long as the gas stays a gas. When gases get very cold, they turn into liquids before they reach absolute zero. So, a gas can't actually shrink to zero volume.
The idea of absolute zero was first talked about by William Thomson, 1st Baron Kelvin (who became Lord Kelvin) in 1848. The Kelvin temperature scale starts at absolute zero.
How Does it Work? (Kinetic Theory)
The kinetic theory of gases helps us understand Charles' law. This theory looks at gases from a tiny, microscopic level. It says that gas is made of many tiny particles (molecules) that are always moving around.
Temperature is related to how fast these gas particles are moving. The hotter the gas, the faster its particles move. When particles move faster, they hit the walls of their container more often and with more force. If the pressure is kept the same, the gas needs more space (volume) for these faster-moving particles.
So, when you heat a gas, its particles move faster, and they need more room. This makes the gas expand, which is exactly what Charles' law describes!
See also