Boyle's law facts for kids
Boyle's Law is a super important idea in physics and chemistry that helps us understand how gases behave. It's also sometimes called the Boyle–Mariotte Law or just Mariotte's Law (especially in France).
Simply put, Boyle's Law tells us that if you squeeze a gas into a smaller space, its pressure will go up. And if you give a gas more space, its pressure will go down. This is true as long as the amount of gas and its temperature stay the same.
Contents
What is Boyle's Law?
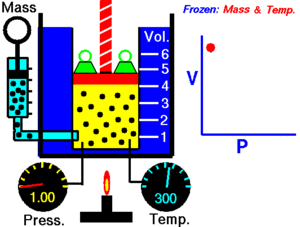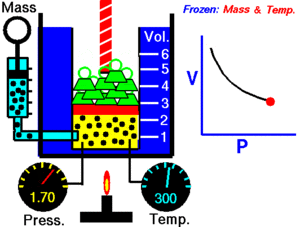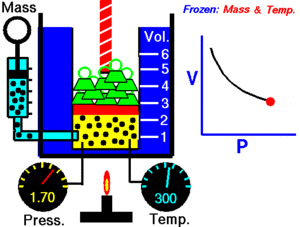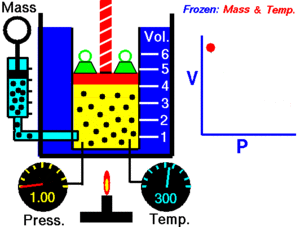
Boyle's Law explains the relationship between the pressure and volume of a gas. It says that for a fixed amount of gas at a constant temperature, the pressure and volume are inversely proportional. This means they move in opposite directions.
If you make the volume of a gas smaller, its pressure will increase. If you make the volume larger, its pressure will decrease. Think of it like a seesaw: when one side goes up, the other goes down.
How Does it Work?
Imagine you have a container filled with gas, like air in a syringe. The gas is made of tiny particles that are constantly moving around and bumping into the walls of the container. These bumps create pressure.
When you push the plunger of the syringe in, you make the space (volume) smaller. Now, the same number of gas particles are squished into a tighter area. This means they hit the walls of the syringe more often. More collisions mean higher pressure!
Real-Life Examples
You can see Boyle's Law in action all around you:
- When you inflate a bicycle tire, you're forcing air into a smaller space, which increases the pressure inside the tire.
- A scuba diver's lungs are affected by pressure changes underwater. As a diver goes deeper, the water pressure increases, which can decrease the volume of air in their lungs.
- When you press down on a pump to inflate a ball, you're reducing the volume of air inside the pump, which increases its pressure and pushes air into the ball.
Who Discovered Boyle's Law?
The law is named after Robert Boyle, an Irish scientist. He discovered this important relationship in 1662 through experiments.
Later, in 1679, a French physicist named Edme Mariotte independently found the same law. That's why it's sometimes called the Boyle–Mariotte Law.
See also
 In Spanish: Ley de Boyle-Mariotte para niños
In Spanish: Ley de Boyle-Mariotte para niños
 | Jackie Robinson |
 | Jack Johnson |
 | Althea Gibson |
 | Arthur Ashe |
 | Muhammad Ali |