Absolute zero facts for kids
Absolute zero is the coldest possible temperature. At this point, the tiny particles (like molecules and atoms) that make up everything are at their lowest energy level.
You might think that at absolute zero, particles stop moving completely. But this isn't quite right! In the world of quantum physics, particles always have a tiny bit of energy, even when they are as cold as can be. This is called "zero-point energy." It's because of a rule called Heisenberg's uncertainty principle. This rule says you can't know both a particle's exact location and its exact speed at the same time. So, a particle can never be perfectly still, because then we would know both its position and its speed exactly.
Contents
Getting Super Cold
Scientists have managed to get very, very close to absolute zero. The record is just a tiny fraction of a degree above it. It's incredibly hard to reach absolute zero because anything that touches the super-cold object would warm it up. Scientists use special tools like lasers to slow down atoms and cool things to these extreme temperatures.
How We Measure It
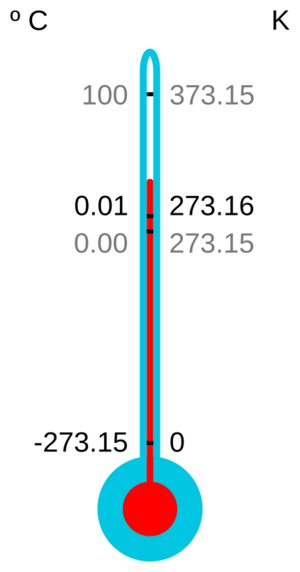
Absolute zero is the starting point for some temperature scales. On the kelvin scale, absolute zero is 0 K. On the Rankine scale, it's 0 °R.
Other common scales define absolute zero differently:
- On the Celsius scale, absolute zero is −273.15 °C.
- On the Fahrenheit scale, it's −459.67 °F.
At this extreme cold, the pressure of particles would be zero. The temperature simply cannot go any lower.
What Happens at Absolute Zero?
When an object gets very close to absolute zero, it becomes super good at conducting electricity. This means electricity can flow through it almost perfectly, with no measurable resistance. This amazing effect is called superconductivity.
The Laws of Thermodynamics
The Third Law of Thermodynamics is a scientific rule that tells us it's impossible to ever reach absolute zero perfectly. We can get very, very close, but never exactly there.
The Second Law of Thermodynamics explains why heat engines (like those in cars or old steam trains) can't be 100% efficient. They always have to release some waste heat. An engine's efficiency depends on the temperature difference between its inside and outside. For an engine to be 100% efficient, the outside temperature would need to be absolute zero, which we know isn't possible. So, while you can make an engine more efficient by making the inside hotter or the outside colder, it will never reach 100%.
Related pages
Images for kids
-
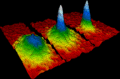
This image shows how a gas of rubidium atoms behaves just above absolute zero. Left: before a special state called a Bose–Einstein condensate appears. Center: just after it appears. Right: after more cooling, showing a nearly pure condensate.
-
Robert Boyle was one of the first scientists to think about the idea of an absolute zero temperature.
See also
 In Spanish: Cero absoluto para niños
In Spanish: Cero absoluto para niños