Heisenberg's uncertainty principle facts for kids
The Heisenberg uncertainty principle is a really important idea in modern physics. It tells us something amazing about tiny particles, like electrons. It says that you can't know everything perfectly about these particles at the same time. For example, you can't know exactly where a particle is and exactly where it's going (its momentum) at the same moment. The more precisely you know one, the less precisely you can know the other.
Famous scientist Albert Einstein thought this "uncertainty" was just about what humans could know. He believed nature itself was certain, even if we couldn't measure it perfectly. But many other scientists disagreed. They think the uncertainty is a basic part of nature itself.
Imagine an electron shot into a big box. You could get a good idea of its future path. But if the box was tiny, you'd know its location much better. Because of that, you'd know less about its exact speed and direction. American physicist Brian Greene used the idea of a moth. In a big closet, a moth flies calmly. But in a small glass jar, it flies wildly. This is a bit like how measuring a tiny particle affects it.
Another cool effect related to this is called quantum tunneling. It's how many electronic devices work! In our daily lives, we can't walk through walls. But electrons can! In the animation you see, a faint white puff appears on the right side of a wall after a big puff hits it from the left. That faint spot shows a photon or other atomic particle tunneling right through the wall!
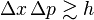
- This math shows that the smallest possible error in knowing a particle's position (x) multiplied by the smallest possible error in knowing its momentum (p) is always at least a certain small number, which involves the Planck constant.
Contents
What is the Uncertainty Principle?
The uncertainty principle is often confused with something called the observer effect. The observer effect means that when you measure something, you might change it. For example, if you check a tire's pressure, some air escapes, changing the pressure slightly.
Heisenberg first thought the uncertainty in quantum particles was due to this observer effect. But now, scientists know it's deeper than that. The uncertainty principle is a basic property of all wave-like systems. Tiny quantum objects act like waves. Because of their wave nature, there's a fundamental limit to how precisely we can know certain pairs of their properties at the same time. It's not just about our tools not being good enough; it's how nature works!
The Idea of "Fuzziness"
The idea of uncertainty came from Werner Heisenberg's work. Before him, Max Planck found that the energy of light is linked to its frequency. He discovered a special number, now called the Planck constant (written as h).
In quantum physics, when you try to describe a particle's position and momentum using special math (called matrices), you find something strange. If you multiply the "momentum matrix" by the "position matrix," you get a different answer than if you multiply the "position matrix" by the "momentum matrix." This difference always involves Planck's constant.
This mathematical discovery means that if you try to measure a particle's position very clearly, its momentum becomes less clear. And if you measure its momentum very clearly, its position becomes less clear. Heisenberg called this "indeterminacy," meaning things are "fuzzy" or not perfectly defined at the quantum level, not just that humans are uncertain about them.
Measuring Position and Momentum
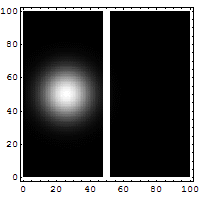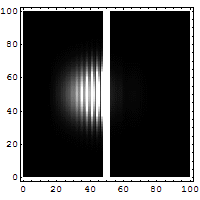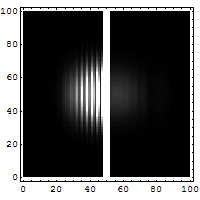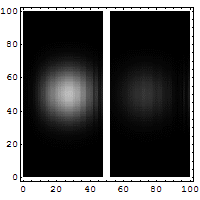
The diagrams below help explain this "fuzziness."
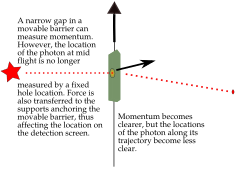
You might think it's easy to know both the exact position and speed of something, like a bullet. If a bullet is stuck in a mountain, its position is known, and its speed is zero. Easy! But if the bullet is flying, it's harder. You might take a fast picture to get its position at one moment. To get its speed, you'd need another measurement, maybe by seeing how far it moves in a certain time. You'd make at least two measurements. For a big bullet, the order doesn't seem to matter, and your measurements don't change the bullet much.
But for tiny things like an electron, it's different. Each measurement you make actually affects the electron. If you measure its position first, you change its momentum. If you measure its momentum first, you change its position. You can't measure one without disturbing the other. The best you can do is to add the smallest possible amount of energy to the electron when you measure it. This minimum amount of energy is linked to the Planck constant.
Uncertainty in Nature
Heisenberg's uncertainty principle was discovered through math, but it's a real fact about nature. It shows up in different ways of describing quantum physics, like in the equations made by Erwin Schrödinger.
Some people believe that everything in nature happens by definite rules, and if we knew everything, we could predict the future perfectly. Others believe that nature is guided by probability, meaning we can only know how things will behave on average. The uncertainty principle supports the idea that at the quantum level, things are truly "fuzzy" or indeterminate, not just that we humans are uncertain.
Uncertainty in Everyday Life
The phrase "quantum leap" or "quantum jump" is often used to mean a huge, sudden change. But in quantum mechanics, it just means an electron moving from one energy level to another around an atom's center. It can be a very small jump!
Sometimes, the word "quantum" is used in product names, like a type of lawnmower engine.
The idea that observing something can change it is also used in everyday life. For example, an anthropologist studying a remote village might change how the people act just by being there and watching them. This is an example of the Observer effect.
The uncertainty principle tells us there's a fundamental limit to how precisely we can measure certain pairs of properties of tiny particles. The observer effect is a broader idea that our act of watching or measuring can change what we're looking at, sometimes in big ways.
Images for kids
See also
 In Spanish: Relación de indeterminación de Heisenberg para niños
In Spanish: Relación de indeterminación de Heisenberg para niños


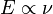 ,
,