Second law of thermodynamics facts for kids
The second law of thermodynamics is a basic rule about how energy and matter behave. It says that when energy changes form, or when matter moves freely, the amount of entropy (which means disorder or randomness) in a system tends to increase. Think of it like your messy room – it usually gets messier on its own, not tidier!
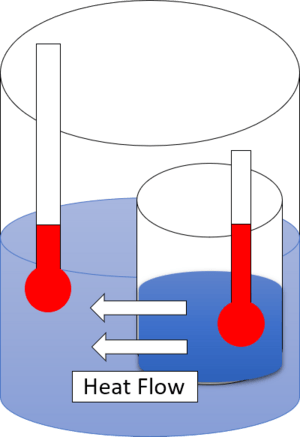
This law means that differences in temperature, pressure, and density will naturally try to spread out and become equal over time. For example, hot things cool down, and cold things warm up until they reach the same temperature. However, gravity still affects density and pressure, so things at the bottom will usually be denser and have more pressure than things at the top.
Entropy is a way to measure how much matter and energy have spread out into all the places they can reach.
One simple way to understand the second law, explained by Rudolf Clausius, is:
It is impossible to build a machine that only moves heat from a colder place to a hotter place.
This means heat always flows from hot to cold on its own. You need to add energy to make it go the other way, like in a refrigerator.
Another way Clausius put it is:
Heat cannot move by itself from a colder object to a hotter object.
Lord Kelvin also had a similar idea:
You cannot create a machine that only takes heat from one source and turns it completely into useful work.
The second law mostly applies to large systems. It describes what usually happens in a system where no energy or matter can get in or out. The bigger the system, the more certain this law is.
What the Second Law Means
In simple terms, the second law tells us that temperature differences between things that are touching will naturally try to become equal. You can get work (useful energy) from these differences, but some energy will always be lost as heat, and entropy will go up.
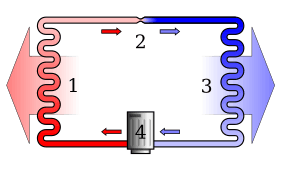
Imagine an isolated system, like a sealed box. Any differences in pressure, density, or temperature inside that box will try to even out. For example, if one part is hot and another is cold, the heat will spread until everything is the same temperature. A heat engine is a machine that uses these temperature differences to do useful work, like a car engine.
Images for kids
See also
 In Spanish: Segundo principio de la termodinámica para niños
In Spanish: Segundo principio de la termodinámica para niños
 | DeHart Hubbard |
 | Wilma Rudolph |
 | Jesse Owens |
 | Jackie Joyner-Kersee |
 | Major Taylor |