Third law of thermodynamics facts for kids
The third law of thermodynamics is a cool rule about how cold things can get. It tells us what happens when something reaches the coldest possible temperature, called absolute zero.
Imagine atoms as tiny, tiny balls that are always wiggling and moving. The third law says that if an object could reach absolute zero, its atoms would stop moving completely. They would be perfectly still!
Absolute zero is super, super cold. It's 0 K. That's the same as about -273.15 C or -459.67 °F.
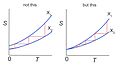
Another important part of the third law is about something called entropy. Entropy is like a measure of how messy or spread out the energy is in something. The law says that at absolute zero, the entropy of a perfectly organized substance (like a crystal) becomes zero. This means there's no messiness or randomness at all.
Why We Can't Reach Absolute Zero
Scientists have tried very hard to reach absolute zero, but it's impossible with today's technology. We can get incredibly close, but never exactly there.
One reason is that most gases turn into liquids or even solids long before they get that cold. Their tiny molecules stop acting like a gas. So far, no one has found a way to cool things down to that exact point. Maybe in the future, new discoveries will change this!
Related Laws of Thermodynamics
Images for kids
See also
 In Spanish: Tercer principio de la termodinámica para niños
In Spanish: Tercer principio de la termodinámica para niños
 | Dorothy Vaughan |
 | Charles Henry Turner |
 | Hildrus Poindexter |
 | Henry Cecil McBay |