Ideal gas law facts for kids
The ideal gas law is a special equation. It helps us understand how a perfect, imaginary gas behaves. This law was first created by a scientist named Benoît Paul Émile Clapeyron in 1834.
It shows how the pressure, volume, and temperature of a gas are all connected. Think of it like a secret code for gases!
Contents
What is the Ideal Gas Law?
The ideal gas law uses a simple formula to describe how gases act. It helps scientists and engineers predict what will happen to a gas if you change its conditions. For example, if you heat a gas, its pressure might go up. This law helps explain why.
Understanding the Formula
The main formula for the ideal gas law looks like this: 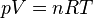
Let's break down what each letter means:
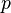 stands for the pressure of the gas. This is how much force the gas molecules push on their container.
stands for the pressure of the gas. This is how much force the gas molecules push on their container.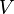 stands for the volume of the gas. This is how much space the gas takes up.
stands for the volume of the gas. This is how much space the gas takes up.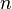 stands for the number of moles of gas. A mole is just a way to count a very large number of tiny gas particles.
stands for the number of moles of gas. A mole is just a way to count a very large number of tiny gas particles.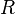 is the universal gas constant. It's a special number that never changes. It helps make the equation work.
is the universal gas constant. It's a special number that never changes. It helps make the equation work.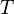 is the absolute temperature of the gas. This is how hot or cold the gas is, measured on a special scale called the Kelvin scale.
is the absolute temperature of the gas. This is how hot or cold the gas is, measured on a special scale called the Kelvin scale.
What is an Ideal Gas?
An "ideal gas" is a made-up gas. It's a perfect model that scientists use. In this perfect model, the gas particles:
- Are very tiny, like tiny dots.
- Don't attract or repel each other. They don't stick together or push each other away.
- Only bump into each other and the container walls. These bumps are called collisions.
Real gases are not perfectly ideal. But for many everyday situations, they act very much like an ideal gas. This makes the ideal gas law very useful for understanding real gases too.
Why is it Useful?
The ideal gas law is used in many places. For example:
- It helps engineers design things like propane tanks. They need to know how much pressure the gas will create inside the tank, especially on a hot day. If the temperature goes up, the pressure inside the tank also goes up. The law helps them make sure the tank is strong enough.
- It's used in weather forecasting to understand how air pressure changes.
- It helps scientists study how gases behave in engines and other machines.
The law helps us predict how gases will act when their conditions change. This is important for safety and for making new technologies.
Images for kids
-
Molecular collisions within a closed container (a propane tank) are shown (right). The arrows represent the random motions and collisions of these molecules. The pressure and temperature of the gas are directly proportional: As temperature increases, the pressure of the propane gas increases by the same factor. A simple consequence of this proportionality is that on a hot summer day, the propane tank pressure will be elevated, and thus propane tanks must be rated to withstand such increases in pressure.
See also
 In Spanish: Ley de los gases ideales para niños
In Spanish: Ley de los gases ideales para niños
 | Valerie Thomas |
 | Frederick McKinley Jones |
 | George Edward Alcorn Jr. |
 | Thomas Mensah |