Selenium trioxide facts for kids
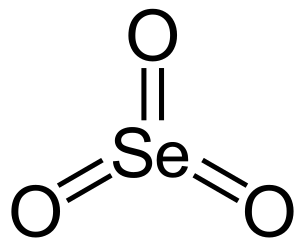
Selenium trioxide is a special kind of chemical compound. You can also call it selenium(VI) oxide or selenic oxide. Its chemical formula is SeO3. This means it's made of one selenium atom and three oxygen atoms joined together. In this compound, selenium is in a specific form called its +6 oxidation state, which describes how many electrons it has shared or gained. It also contains parts called oxide ions, which are oxygen atoms with an electrical charge.
What it's like
Selenium trioxide is a white solid, like a white powder or crystal. It can easily turn into a gas, which means it "evaporates" or "sublimes" quickly. It also dissolves very well in water.
However, selenium trioxide is not very stable. If you heat it up, it breaks apart into two simpler substances: selenium dioxide and oxygen gas. It is also a strong oxidizing agent. This means it can take electrons from other chemicals, causing them to change. It's similar to how other selenates (compounds containing selenium and oxygen) act. When selenium trioxide dissolves in water, it forms a strong acid called selenic acid.
How it's made
Scientists can make selenium trioxide in a few ways. One way is to mix a chemical called potassium selenate with sulfur dioxide. Another method involves taking water out of selenic acid. They do this by using a very strong dessicant, which is a substance that absorbs water. An example of such a dessicant is phosphorus(V) oxide.
Related topics
See also
 In Spanish: Trióxido de selenio para niños
In Spanish: Trióxido de selenio para niños