Selenate facts for kids
Quick facts for kids Selenate |
|
|---|---|
 |
|
| IUPAC name | selenate |
| Other names | selenate ion |
| Identifiers | |
| CAS number | |
| PubChem | |
| SMILES | [O-][Se+2]([O-])([O-])[O-] |
| Properties | |
| Molecular formula | |
| Molar mass | 0 g mol-1 |
| Conjugate acid | Hydrogen selenate |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
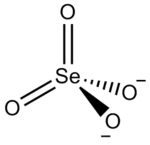
A selenate is a special kind of ion, which is an atom or group of atoms that has an electric charge. Its chemical formula is SeO42-. This means it has one selenium atom and four oxygen atoms, and it carries a negative charge of two. Selenates are known for dissolving very easily in water. They are also strong oxidizing agents, which means they can take electrons from other substances in a chemical reaction. When selenate is in a very acidic environment, it can change into selenic acid. A common example of a selenate is Sodium selenate.
Related Topics
See also
 In Spanish: Selenato para niños
In Spanish: Selenato para niños
Black History Month on Kiddle
Renowned African-American Artists:
 | Selma Burke |
 | Pauline Powell Burns |
 | Frederick J. Brown |
 | Robert Blackburn |

All content from Kiddle encyclopedia articles (including the article images and facts) can be freely used under Attribution-ShareAlike license, unless stated otherwise. Cite this article:
Selenate Facts for Kids. Kiddle Encyclopedia.